
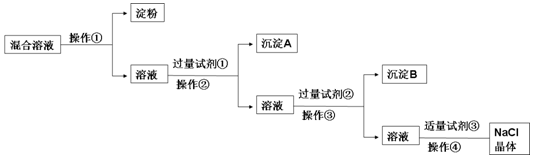
���� ���岻������Ĥ��������Ϊ��������ȥNa2SO4��CaCl2���ɷֱ����BaCl2��Na2CO3����ȥ�����к��е�Ca2+��SO42-���������ʵķ������������BaCl2��ȥ����������ӣ��ټ������Na2CO3��ȥ�������ӣ������Լ���ΪBaCl2��������Ϊ���ˣ�����AΪ���ᱵ���Լ���ΪNa2CO3��������Ϊ���ˣ�����BΪ̼��ƺ�̼�ᱵ���Լ���Ϊ���ᣬ��������ɳ�ȥ������Na2CO3����������ᾧ�ɵõ�NaCl���壬�Դ˽����⣮
��� �⣺��1�������Ϸ�����֪�Լ���ΪBaCl2���Լ���ΪHCl���ʴ�Ϊ��BaCl2��HCl��
��2���ж��Լ����ѹ����ķ����Ǿ��ã����ϲ���Һ�еμ������Ȼ�����Һ��û�а�ɫ����������˵���Ȼ�����Һ�ѹ�����
�ʴ�Ϊ�����ã����ϲ���Һ�еμ������Ȼ�����Һ���ް�ɫ����������˵���Ȼ�����Һ�ѹ�����
��3������Ϊ���壬����ֱ���ϴ�������Ĥ����������ӿ�����Ĥ���ʴ�Ϊ�����ܣ��ܣ�
��4������������Һ�õ����壬���������ᾧ�ķ������ʴ�Ϊ�������ᾧ��
��5��Ӧ����500mL��NaCl�����ʵ���n=cV=0.5L��1.0mol•L-1=0.5mol��NaCl������Ϊ0.5mol��58.5g/mol=29.25g������ƽ�ľ�ȷ��Ϊ0.1g������������ƽʵ�ʳ�ȡNaCl���������Ϊ29.3g�����Ʋ����м��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣬��ȴ��ת�Ƶ�100mL����ƿ�У����ò���������������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�������Ҫ������Ϊ��������ƽ��ҩ�ס��ձ�����������100mL����ƿ����ͷ�ιܣ�
������ˮϴ������ƿ��û�к����Ӱ�죬����ʱ��Ҫ��������ˮ���ݣ�
��ת����Һ�����г���©Һʹ���ʼ��٣�Ũ��ƫ�ͣ�
���ܽ��δ����ȴ��ת�Ƶ�����ƿ��ʹ��Һ�����ƫС��Ũ��ƫ�ߣ�
�ܶ���ʱ��������ƿ�̶��ߣ���Һ��Һ����ڿ̶��ߣ����ƫС��Ũ��ƫ�ߣ�
��ҡ�Ⱥ���Һ�潵�ͣ��ټ�����ˮ��ʹ��Һ���ƫ��Ũ��ƫС��
�ʴ�Ϊ��29.3g��500mL����ƿ���������� �ۢܣ�
���� ���⿼�����ʵķ����ᴿ�����Լ���Һ�����ƣ�Ϊ��Ƶ���㣬��Ŀ�Ѷ��еȣ�����ע��Ca2+��SO42-�����ʣ����ճ���ԭ���ᴿʱ���������µ����ʣ�ע�����ʵ����Ⱥ�˳��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
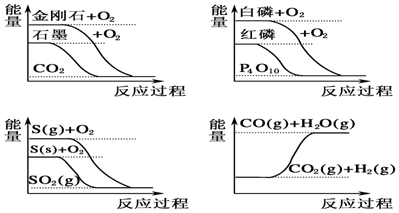
| A�� | CO��g��+H2O��g���TCO2��g��+H2��g����H��0 | |
| B�� | S��g��+O2��g���TSO2��g����H1��S��s��+O2��g���TSO2��g����H2�����H1����H2 | |
| C�� | ���ױȺ����ȶ� | |
| D�� | ʯīת��Ϊ���ʯ�����ȷ�Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 0.1 mol/L�ģ�NH4��2SO4��Һ��c��SO42-����c��NH4+����c��H+����c��OH-�� | |
| B�� | 0.1 mol/L��NaHCO3��Һ��c��Na+��=c��HCO3-��+c��H2CO3��+2c��CO32-�� | |
| C�� | ��0.2 mol/L NaA��Һ��0.1 mol/L�����������������Һ��c��Na+��+c��H+��=c��A-��+c��Cl-�� | |
| D�� | 0.02mol/L CH3COOH��Һ��0.01 mol/L NaOH��Һ��������������Һ��2c��H+��+c��CH3COOH��=c��CH3COO-��+2c��OH-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Na+ 1s22s22p63s2 | B�� | F 1s22s22p5 | ||
| C�� | O2- 1s22s22p6 | D�� | Ar 1s22s22p63s23p6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
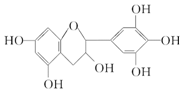
| A�� | ����ʽΪC15H16O7 | |
| B�� | 1 mol������A��һ���������������6 mol H2�ӳ� | |
| C�� | �������Ķ�����A�ֱ��������Ľ����ƺ��������Ʒ�Ӧ���Ľ����ƺ��������Ƶ����ʵ���֮��Ϊ1��1 | |
| D�� | 1 mol������A��������Ũ��ˮ��Ӧ���������Br2 4 mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 +
+ ��
�� +H2O��
+H2O��| A�� | 1molCPAE��1mol������ֱ���������NaOH��Һ��Ӧ�����������3molNaOH | |
| B�� | �뱽�Ҵ���Ϊͬ���칹��ķ������ʹ���9�� | |
| C�� | FeCl3��Һ���������CPAE | |
| D�� | ������ɷ����ۺϷ�Ӧ������������к���3�ֹ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1mol�����С�C�TC���ĸ���Ϊ3 NA | |
| B�� | ���³�ѹ�£�22.4L���������еĹ��ۼ���ĿΪ10NA | |
| C�� | ��״���£�1L������ȼ�պ����ɵ���̬����ķ�����Ϊ$\frac{5}{22.4}$ NA | |
| D�� | 2.8g��ϩ�ͱ�ϩ�Ļ������������̼ԭ����Ϊ0.2NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | V1=20mL��V2��20mL | |
| B�� | A��֮����B��֮ǰ����Һ�У�һ���ǣ�c��X- ����c�� Na+����c��H+����c��OH- �� | |
| C�� | ��V1=10mL��C�����Һ�У�һ���ǣ�c��HY ��-c�� Y-��=2[c��OH- ��-c��H+��] | |
| D�� | D���Ժ����Һ�У�һ���ǣ�c�� Na+����c��OH- ����c��Y- ����c��H+�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com