
| ��� | �� | �� | �� | �� | �� | �� | �� |
| ���� | �ƾ� | �� | ��� | ʳ�� | ͭ���� | �������� | �մ� |
| ��Ҫ �ɷ� |
CH3CH2OH | CH3COOH | NaOH | NaCl | Cu | SO2 | Na2CO3 |
| ||
| ||
 ��Cu���ϼ����ߣ���0�����ߵ�+2�ۣ�SԪ�ػ��ϼ۽��ͣ���+6�۽��͵�+4�ۣ����ݷ���ʽÿ��Ӧ1molͭת��2mol���ӣ�������ת��0.1molʱ����Ӧ��ͭΪ0.05mol������Ϊ3.2g���ʴ�Ϊ��
��Cu���ϼ����ߣ���0�����ߵ�+2�ۣ�SԪ�ػ��ϼ۽��ͣ���+6�۽��͵�+4�ۣ����ݷ���ʽÿ��Ӧ1molͭת��2mol���ӣ�������ת��0.1molʱ����Ӧ��ͭΪ0.05mol������Ϊ3.2g���ʴ�Ϊ��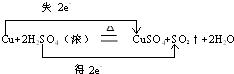 ��3.2��
��3.2��

 ��Ǭ����������ҵ���ּ����ӱ����������ϵ�д�
��Ǭ����������ҵ���ּ����ӱ����������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��� | �� | �� | �� | �� | �� | �� | �� |
| ���� | �̷� | �� | �մ� | �ƾ� | ͭ���� | ���� | �ռ� |
| ��Ҫ�ɷ� | FeSO4 | CH3COOH | Na2CO3 | CH3CH2OH | Cu | C12H22O11 | NaOH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�츣��ʡ��һ��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ������
(11��)�±������������г��������ʣ������г������ǵģ���Ҫ���ɷ֡�
|
��� |
�� |
�� |
�� |
�� |
�� |
�� |
�� |
|
���� |
�ƾ� |
���� |
��� |
ʳ�� |
ͭ���� |
�������� |
�մ� |
|
��Ҫ �ɷ� |
CH3CH2OH |
CH3COOH |
NaOH |
NaCl |
Cu |
SO2 |
Na2CO3 |
��1������Ա��Т١��ߵ���Ҫ�ɷֽ��з��ࣨ���ţ���
�����ε��� �����ڵ���ʵ��� �����ڷǵ���ʵ��� ��
��2��д�������ڵ�ˮ��Һ��߷�Ӧ�Ļ�ѧ����ʽ ��
����������߷�Ӧ�����ӷ���ʽ ��
��ijͬѧ�âݺ�Ũ���Ṳ�����Ʊ��ޣ���ѧ����ʽΪ��
Cu+2H2SO4��Ũ�� CuSO4+SO2��+2H2O
CuSO4+SO2��+2H2O
��������ת�Ƶķ������Ŀ��������ת��0.1molʱ�����Ļ�ԭ��������Ϊ g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��㶫ʡ��һ��ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��6�֣��±������������г��������ʣ������г������ǵ�һ����Ҫ�ɷ֣�

����Ա��Т�~�ߵ���Ҫ�ɷֽ��з��ࣨ���ţ�
�����ε���_________�����ڵ���ʵ���________�����ڷǵ���ʵ���_______��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com