



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ʡȪ������У2010�������ѧ����ĩ��������ѧ�� ���ͣ������
ij����С��ͬѧ����ͼװ�ý���ʵ�飬�Իش��������⣺
��.��ͼ1��ʵ��װ�ý���ʵ�顣
��1������ʼʱ����K2���պϿ���K1�����װ��Ϊ ����װ�����ƣ���
��2������ʼʱ����K1���պϿ���K2����
��У2010�������ҵ����ĩ������ѧ�Ծ� ��7ҳ ����12ҳ��
��U�����ܷ�Ӧ�����ӷ���ʽΪ 
�ڶ�������ʵ�飬����˵����ȷ���� ������ţ���
| A����Һ��Na+��B���ƶ� |
| B����A�����ݳ���������ʹʪ���KI������ֽ���� |
| C����Ӧһ��ʱ������������ɻָ������ǰ����ʵ�Ũ�� |
| D������״���´�A���ݳ�2.24L���壬�����·��ͨ���ĵ�����Ŀ�Դ���0.2NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij����С��ͬѧ����ͼװ�ý���ʵ�飬�Իش��������⣺
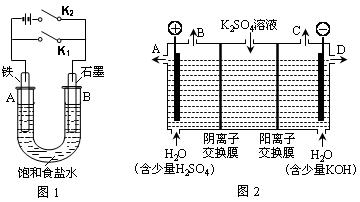
��.��ͼ1��ʵ��װ�ý���ʵ�顣
��1������ʼʱ����K2���պϿ���K1�����װ��Ϊ ����װ�����ƣ���
��2������ʼʱ����K1���պϿ���K2����
��У2010�������ҵ����ĩ������ѧ�Ծ� ��7ҳ ����12ҳ��
��U�����ܷ�Ӧ�����ӷ���ʽΪ ![]()
�ڶ�������ʵ�飬����˵����ȷ���� ������ţ���
A. ��Һ��Na+��B���ƶ�
B. ��A�����ݳ���������ʹʪ���KI������ֽ����
C. ��Ӧһ��ʱ������������ɻָ������ǰ����ʵ�Ũ��
D. ����״���´�A���ݳ�2.24L���壬�����·��ͨ���ĵ�����Ŀ�Դ���0.2NA
��.��ͼ2��ʵ��װ�ý���ʵ�顣
��3����С��ͬѧ��Ϊ�����ģ�ҵ�����ӽ���Ĥ�����ռ�ķ�������ô������������ͼ2װ�õ���������Һ����ȡ������������������������أ������ڵ������ӽ���Ĥֻ����������ͨ���������ӽ���Ĥֻ����������ͨ������
�ٸõ��۵�������ӦʽΪ ����ʱͨ�������ӽ���Ĥ�������� ������ڡ�����С�ڡ����ڡ���ͨ�������ӽ���Ĥ����������
���Ƶõ�����������Һ�ӳ��� ���A������B������C������D����������
��ͨ�翪ʼ������������Һ��pH�����������ԭ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij����С��ͬѧ����ͼװ�ý���ʵ�飬�Իش��������⣺
 |
��.��ͼ1��ʵ��װ�ý���ʵ�顣
��1������ʼʱ����K2���պϿ���K1�����װ��Ϊ ����װ�����ƣ���
��2������ʼʱ����K1���պϿ���K2����
��У2010�������ҵ����ĩ������ѧ�Ծ� ��7ҳ ����12ҳ��
��U�����ܷ�Ӧ�����ӷ���ʽΪ
�ڶ�������ʵ�飬����˵����ȷ���� ������ţ���
A. ��Һ��Na+��B���ƶ�
B. ��A�����ݳ���������ʹʪ���KI������ֽ����
C. ��Ӧһ��ʱ������������ɻָ������ǰ����ʵ�Ũ��
D. ����״���´�A���ݳ�2.24L���壬�����·��ͨ���ĵ�����Ŀ�Դ���0.2NA
��.��ͼ2��ʵ��װ�ý���ʵ�顣
��3����С��ͬѧ��Ϊ�����ģ�ҵ�����ӽ���Ĥ�����ռ�ķ�������ô������������ͼ2װ�õ���������Һ����ȡ������������������������أ������ڵ������ӽ���Ĥֻ����������ͨ���������ӽ���Ĥֻ����������ͨ������
�ٸõ��۵�������ӦʽΪ ����ʱͨ�������ӽ���Ĥ�������� ������ڡ�����С�ڡ����ڡ���ͨ�������ӽ���Ĥ����������
���Ƶõ�����������Һ�ӳ��� ���A������B������C������D����������
��ͨ�翪ʼ������������Һ��pH�����������ԭ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡȪ������У2010�������ѧ����ĩ���� ���ͣ������
ij����С��ͬѧ����ͼװ�ý���ʵ�飬�Իش��������⣺
 |
��.��ͼ1��ʵ��װ�ý���ʵ�顣
��1������ʼʱ����K2���պϿ���K1�����װ��Ϊ ����װ�����ƣ���
��2������ʼʱ����K1���պϿ���K2����
��У2010�������ҵ����ĩ������ѧ�Ծ� ��7ҳ ����12ҳ��
��U�����ܷ�Ӧ�����ӷ���ʽΪ
�ڶ�������ʵ�飬����˵����ȷ���� ������ţ���
A. ��Һ��Na+��B���ƶ�
B. ��A�����ݳ���������ʹʪ���KI������ֽ����
C. ��Ӧһ��ʱ������������ɻָ������ǰ����ʵ�Ũ��
D. ����״���´�A���ݳ�2.24L���壬�����·��ͨ���ĵ�����Ŀ�Դ���0.2NA
��.��ͼ2��ʵ��װ�ý���ʵ�顣
��3����С��ͬѧ��Ϊ�����ģ�ҵ�����ӽ���Ĥ�����ռ�ķ�������ô������������ͼ2װ�õ���������Һ����ȡ������������������������أ������ڵ������ӽ���Ĥֻ����������ͨ���������ӽ���Ĥֻ����������ͨ������
�ٸõ��۵�������ӦʽΪ ����ʱͨ�������ӽ���Ĥ�������� ������ڡ�����С�ڡ����ڡ���ͨ�������ӽ���Ĥ����������
���Ƶõ�����������Һ�ӳ��� ���A������B������C������D����������
��ͨ�翪ʼ������������Һ��pH�����������ԭ�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com