
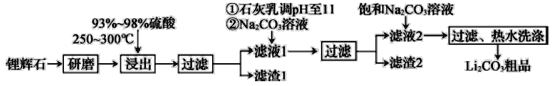
����Ŀ����ҵ����﮻�ʯΪԭ������̼��﮵IJ��ֹ�ҵ������ͼ��ʾ��
��֪��
��﮻�ʯ����Ҫ�ɷ�ΪLi2O��Al2O3��4SiO2�����к�����Ca��MgԪ�ء�
��Li2O��Al2O3��4SiO2+H2SO4(Ũ)![]() Li2SO4+Al2O3��4SiO2��H2O��
Li2SO4+Al2O3��4SiO2��H2O��
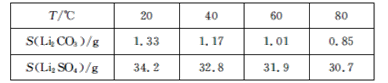
��ijЩ���ʵ��ܽ��(S)���±���ʾ��
�ش��������⣺
��1��������1�з����Al2O3������������ʾ��
![]()
д�����ɳ��������ӷ���ʽ��___��
��2����֪����2����Ҫ�ɷ���Mg(OH)2��CaCO3������Һ1�м���ʯ�����������___(���û�ѧƽ��ԭ������)��
��3�����һ�������У�������ˮϴ������������___��
��4����ҵ�ϣ���Li2CO3��Ʒ�Ʊ��ɸߴ�Li2CO3�IJ��ֹ������£�
a.��Li2CO3�������������۵�����Һ��LiOH��Һ������Һ������������ѡ������Ĥ�������ö��Ե缫��⡣
b.������LiOH��Һ�м�������NH4HCO3��Һ�����ȣ����ˡ���ɵøߴ�Li2CO3��
��a�е��ʱ���õ���___(���������ӽ���Ĥ�����������ӽ���Ĥ��)��
�ڵ���LiOH��ҺŨ�������ԭ����___��
b������Li2CO3��Ӧ�Ļ�ѧ����ʽ��___��
��5����������﮵���ܷ�ӦΪFePO4+Li![]() LiFePO4������еĹ������ʿɴ���Li+��д���õ�طŵ�ʱ��������Ӧ��___��
LiFePO4������еĹ������ʿɴ���Li+��д���õ�طŵ�ʱ��������Ӧ��___��
���𰸡�Al3����3NH3��H2O===Al(OH)3����3NH4�� Ca(OH)2 ![]() Ca2++2OH-��Mg2+��OH-�������Ksp��С��Mg(OH)2����������ƽ�����ƣ�����Mg(OH)2���� Li2CO3���ܽ�����¶����߶���С �����ӽ���Ĥ ���������ӷŵ磬������������ƶ� 2LiOH+NH4HCO3
Ca2++2OH-��Mg2+��OH-�������Ksp��С��Mg(OH)2����������ƽ�����ƣ�����Mg(OH)2���� Li2CO3���ܽ�����¶����߶���С �����ӽ���Ĥ ���������ӷŵ磬������������ƶ� 2LiOH+NH4HCO3![]() Li2CO3�� +2H2O+NH3�� FePO4��Li+��e-===LiFePO4
Li2CO3�� +2H2O+NH3�� FePO4��Li+��e-===LiFePO4
��������
�ɹ�ҵ����ͼ��֪��﮻�ʯ������ĥ�����ȡ��ữ�õ������Ե�����ﮡ��������Ͳ��ܵĶ������裬���˵õ���Һ1�ͺ��ж������������1������Һ1�м���ʯ�������pHֵ���ټ���̼������Һ����������Һ��Ca2+��OH-��Ũ�ȣ�ʹMg��OH��2��CaCO3�������������Ա��γ�����2������Һ2�м��뱥��̼������Һ����Ӧ����̼��﮳��������ˡ���ˮϴ�ӵ�ԭ����Li2CO3���ܽ�����¶����߶���С���ɼ���Li2CO3����ʧ���õ����ղ���̼��ﮣ��ݴ˷������
(1)����֪��Ϣ�ٿ�֪����1�к����������Ͷ������裬����������������������������������������������������ܽ�����1�����˺�õ�����������Ȼ�������Һ������Һ��ͨ�������İ������������������������������������ɵõ������������ɳ��������ӷ���ʽΪAl3����3NH3��H2O===Al(OH)3����3NH4�����ʴ�Ϊ��Al3����3NH3��H2O===Al(OH)3����3NH4����
(2)ʯ�����д����������Ƶ��ܽ�ƽ��Ca(OH)2 ![]() Ca2++2OH-��Mg2+��OH-�������Ksp��С��Mg(OH)2����������ƽ�����ƣ�����Mg(OH)2����������������̼����������̼��Ƴ������ʴ�Ϊ��Ca(OH)2
Ca2++2OH-��Mg2+��OH-�������Ksp��С��Mg(OH)2����������ƽ�����ƣ�����Mg(OH)2����������������̼����������̼��Ƴ������ʴ�Ϊ��Ca(OH)2 ![]() Ca2++2OH-��Mg2+��OH-�������Ksp��С��Mg(OH)2����������ƽ�����ƣ�����Mg(OH)2������
Ca2++2OH-��Mg2+��OH-�������Ksp��С��Mg(OH)2����������ƽ�����ƣ�����Mg(OH)2������
(3)���ݱ������ݿ��ж�Li2CO3���ܽ�����¶����߶���С��������һ�������У�������ˮϴ�������Լ��ٹ����ܽ����ɵ���ʧ���ʴ�Ϊ��Li2CO3���ܽ�����¶����߶���С��
(4)�ٵ���������ʧȥ���ӷ���������Ӧ�����������ӷŵ磬�������������ӣ���������������ƶ�����a�е��ʱ���õ��������ӽ���Ĥ���ʴ�Ϊ�������ӽ���Ĥ��
�������õ����ӣ�����Һ�е������ӷŵ磬�������������ӷŵ磬�������������ӣ���������������ƶ������Ե���LiOH��ҺŨ��������ԭ���غ���ж�LiOH��Һ�м�������NH4HCO3��Һ�����ȣ����õ��ߴ�Li2CO3��а�����ˮ���ɣ���Ӧ�Ļ�ѧ����ʽΪ2LiOH+NH4HCO3![]() Li2CO3��+2H2O+NH3�����ʴ�Ϊ�����������ӷŵ磬������������ƶ���2LiOH+NH4HCO3
Li2CO3��+2H2O+NH3�����ʴ�Ϊ�����������ӷŵ磬������������ƶ���2LiOH+NH4HCO3![]() Li2CO3��+2H2O+NH3����
Li2CO3��+2H2O+NH3����
(5)����ԭ��صĹ���ԭ���������Ϸ�����ԭ��Ӧ���õ����ӣ����FePO4�������Ϸ�����Ӧ����������ӦʽΪFePO4��Li����e��===LiFePO4���ʴ�Ϊ��FePO4��Li+��e-===LiFePO4��


 ѧ���쳵�����ּ��ں�����ҵϵ�д�
ѧ���쳵�����ּ��ں�����ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��H2��g����I2��g����H2��g����I2��s)�Լ�HI��g���������ߵ���ͼ��ʾ�������жϴ�����ǣ� ��
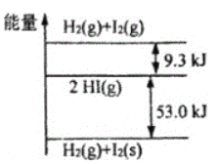
A.1molH2��g����1molI2��g���������ܺ���2molHI��g��������
B.I2��g����I2��s��+QkJ��Q>0��
C.H2��g��+I2��g����2HI��g��+9.3kJ
D.H2��g��+I2��s����2HI��g��+53.0kJ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������������������ķ�����������ΪԽ��Խ��Ҫ�Ļ��������������⡣������Ҫ�ɶ�������������Ϳ������������������ɡ�
(1)��������β���������γɵ�ԭ��֮һ�������������������ѡ���Դ����������лӷ�������C3H6����ԭβ���е�NO���壬��д���ù��̵Ļ�ѧ����ʽ��__________
(2)���˶������ҹ�������ú��ů�������ķ���Ҳ����������Ҫ��Դ֮һ�����о����ֽ�ú̿��O2/CO2��������ȼ�գ������ܹ�����ȼúʱNO���ŷţ���Ҫ��ӦΪ��
2NO(g)+2CO(g)![]() N2(g)+2CO2(g) ��H
N2(g)+2CO2(g) ��H
���� N2(g)+O2(g)![]() 2NO(g) ��H1= ��180.5 kJmol-1
2NO(g) ��H1= ��180.5 kJmol-1
�� CO(g)![]() C(s)+1/2O2(g) ��H2= ��110.5 kJmol-1
C(s)+1/2O2(g) ��H2= ��110.5 kJmol-1
�� C (s)+O2(g)![]() CO2(g) ��H3= ��393.5 kJmol-1
CO2(g) ��H3= ��393.5 kJmol-1
����H=_____________kJmol-1��
(3)ȼúβ���е�SO2��NaOH��Һ�����γ�NaHSO3��Һ����pHΪ4~7֮��ʱ��⣬��Ԫ����Ǧ�����ϱ���ԭΪNa2S2O4��Na2S2O4�׳Ʊ��շۣ��㷺Ӧ����Ⱦ�ϡ�ӡȾ����ֽ��ʳƷ��ҵ�Լ�ҽѧ�ϡ����ּ���������ĵ绯ѧ������֮һ����д���õ�ⷴӦ�������ĵ缫����ʽ��______________________________________��
(4)SO2�����������������Ͻ��д���������ȡ�����������泥�����SO2�����������ķ�ӦΪ��2SO2(g) +O2(g)![]() 2SO3(g)������T1�桢0.1MPa�����£���һ�ܱ�����ͨ��SO2��O2(����n(SO2):n(O2)=2:1)�������������ѹǿ�뷴Ӧʱ����ͼ��ʾ��
2SO3(g)������T1�桢0.1MPa�����£���һ�ܱ�����ͨ��SO2��O2(����n(SO2):n(O2)=2:1)�������������ѹǿ�뷴Ӧʱ����ͼ��ʾ��
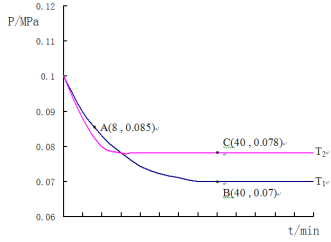
�ٸ÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽ��K=__________________��
��ͼ��A��ʱ��SO2��ת����Ϊ________________
�ۼ���SO2��������Ӧ��ͼ��B���ѹǿƽ�ⳣ��K��_______________(��ƽ���ѹ����ƽ��Ũ�ȼ��㣬��ѹ����ѹ�����ʵ�������)
������T2�棬�����������������²��ѹǿ�ı仯������ͼ��ʾ����T1____T2(����>������<������=��)������C�������Ӧ����vc(��)��A����淴Ӧ����vA(��)�Ĵ�С��ϵΪvc(��) ____vA(��) (����>������<������=��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ұ���ķ���������Ϊԭ����ȡ��ϸ��-���������Ƚ��ͻ�����Ⱦ�ֿ��������Դ�������ʡ���֪���ҵ���Ҫ�ɷ�ΪAl2O3������������SiO2��FeO��Fe2O3�������Ʊ�ʵ��������ͼ��
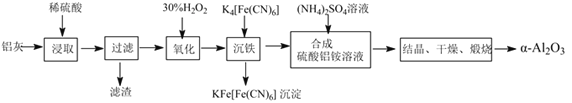
(1)�����������������ᷴӦ�Ļ�ѧ����ʽΪ___��
(2)ͼ��������������Ҫ�ɷ�Ϊ___(�ѧʽ)����30%��H2O2��Һ���������ӷ�Ӧ����ʽΪ2Fe2++H2O2+2H+=2Fe3++2H2O��
(3)��֤������������Һ���Ƿ��������ӵIJ�������Ϊ___��
(4)����������茶��壬��������Ҫ��ӦΪ4[NH4Al(SO4)2��12H2O]![]() 2Al2O3+2NH3��+N2��+5SO3��+3SO2��+53H2O��������������ͨ����ͼ��ʾ��װ�á�
2Al2O3+2NH3��+N2��+5SO3��+3SO2��+53H2O��������������ͨ����ͼ��ʾ��װ�á�

�ټ���ƿ���ռ�����������___(�ѧʽ)��
����������NaHSO3��Һ���յ����ʳ���H2O(g)���__(�ѧʽ)��
��KMnO4��Һ��ɫ(MnO4-��ԭΪMn2+)�����������ӷ�Ӧ����ʽΪ___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ʪ����п�ĵ��Һ��ͬʱ����Cu��CuSO4��������CuCl������ȥCl-�����ͶԵ���Ӱ�죬��Ӧԭ�����£�
Cu(s)+Cu2+(aq)![]() 2Cu+(aq) ��H1=akJ��mol-1
2Cu+(aq) ��H1=akJ��mol-1
Cl��(aq)+Cu+(aq)![]() CuCl(s) ��H2=bkJ��mol-1
CuCl(s) ��H2=bkJ��mol-1
ʵ���õ��ҺpH����Һ�в���c(Cl-)��Ӱ����ͼ��ʾ������˵����ȷ���ǣ� ��
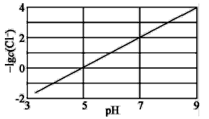
A.����ͭ���ʵ�����������Cl-��ȥ��
B.��ҺpHԽ��Ksp(CuCl)����
C.��Ӧ�ﵽƽ�������c(Cu2+)��c(Cl-)��С
D.![]() Cu(s)+
Cu(s)+![]() Cu2+(aq)+Cl-(aq)
Cu2+(aq)+Cl-(aq)![]() CuCl(s)����H=(a+2b)kJ��mol-1
CuCl(s)����H=(a+2b)kJ��mol-1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ˮMgBr2������������ʵ���Ҳ���þм��Һ��Ϊԭ���Ʊ���ˮMgBr2��װ����ͼ��ʾ����Ҫ�������£�

����1������ƿ��װ��10gþм��150mL��ˮ���ѣ�װ��B�м���15mLҺ�塣
����2������ͨ�����ĵ�����ֱ������ȫ��������ƿ�С�
����3����Ӧ��Ϻ�ָ������£����ˣ���Һת������һ�������ƿ�У���ȴ��0�棬�������壬�ٹ��ˣ��õ������Ѻ��廯þ��Ʒ��
����4���������ñ��ܽ��Ʒ����ȴ��0�棬�������壬���ˣ�ϴ�ӣ��õ������Ѻ��廯þ��������160��ֽ�õ���ˮMgBr2��Ʒ��
��֪��
��Mg��Br2��Ӧ���ҷ��ȣ�MgBr2����ǿ��ˮ�ԡ�
��MgBr2+3C2H5OC2H5![]() MgBr2��3C2H5OC2H5��
MgBr2��3C2H5OC2H5��
�ش��������⣺
��1��װ������ˮ�Ȼ��Ƶ�������___��ʵ���в����ø���Ŀ�����������N2��ԭ����___��
��2���罫װ��B��Ϊװ��C�����ܻᵼ�µĺ����___��
��3��װ����ʹ�����������ܵ�Ŀ����___��
��4������4���ñ��ܽ��Ʒ����ȥ��Ʒ�е�___���ʡ�
��5��Ϊ�ⶨ��Ʒ�Ĵ��ȣ�����EDTA(��дΪY4-)����Һ�ζ�����Ӧ�����ӷ���ʽΪMg2++Y4-=MgY2-��
�ٵζ�ǰ��ϴ�ζ��ܵIJ���������___��
�ڲⶨǰ��ѡ��ȡ0.2500g��ˮMgBr2��Ʒ���ܽ����0.0500mol��L-1��EDTA����Һ�ζ����յ㣬����EDTA����Һ26.50mL��������ˮMgBr2��Ʒ�Ĵ�����___(������������ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����.����һ����Ҫ�Ļ���ԭ�ϣ�ijѧϰС������ȡ������̽�������ʡ���ش�

��1��ʵ������ȡ�����Ļ�ѧ����ʽ��____��
��2����ͼһ�ǽ��а�����Ȫʵ���װ�ã�������Ȫ����ʹ�ձ�����Һ����ɫ��Ϊ��ɫ����ԭ���ǣ��õ��뷽��ʽ��ʾ��____��
��3����С��ͬѧ�������ͼ����ʾ��ʵ��װ�ã����ּг�����δ��������̽�������Ļ�ԭ�Բ�������
��ʵ������Ϊ����ɫCuO��Ϊ��ɫ����ɫ��ˮCuSO4��ĩ��Ϊ��ɫ��ͬʱ����һ����ɫ���壬����������Ⱦ����д��������CuO��Ӧ�Ļ�ѧ����ʽ___��
�ڼ�ʯ�ҵ�������________��
�۸�װ�ô�������ȱ�ݣ���ָ�����ڵ�����______��
��.3.2g Cu��30mL��8mol/L����HNO3��Ӧ������Ļ�ԭ����ΪNO��NO2����Ӧ����Һ������H+Ϊa mol���ٴ�ʱ��Һ��������NO![]() Ϊ_____ mol��
Ϊ_____ mol��
�������ɵ�NO�ڱ�״�������Ϊ_______L�������Ͻ�����ú�a�Ĵ���ʽ��ʾ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ܱ������е�һ����������巢����Ӧ��xA(g)��yB(?) ![]() zC(g)��ƽ��ʱ���A��Ũ��Ϊ0.45 mol/L�������¶Ȳ��䣬���������ݻ�ѹ����ԭ����һ�룬�ٴ�ƽ��ʱ�����A��Ũ������Ϊ0.9 mol/L�������й��ж���ȷ����
zC(g)��ƽ��ʱ���A��Ũ��Ϊ0.45 mol/L�������¶Ȳ��䣬���������ݻ�ѹ����ԭ����һ�룬�ٴ�ƽ��ʱ�����A��Ũ������Ϊ0.9 mol/L�������й��ж���ȷ����
A. �����ʵļ�����һ�����㣺x��y��z
B. ��BΪ���壬ѹ����B�������������ת���ʽ���
C. ����BΪ��������壬ѹ����B������������ı�
D. ѹ��ʱ��ƽ�����淴Ӧ�����ƶ����������淴Ӧ���ʶ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪̼̼���������Ƽ���������ת���ṹ��ʽΪ��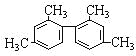 ����������˵����ȷ���ǣ� ��
����������˵����ȷ���ǣ� ��
A.������������9��̼ԭ�Ӵ���ͬһƽ����
B.������������11��̼ԭ�Ӵ���ͬһƽ����
C.������������16��̼ԭ�Ӵ���ͬһƽ����
D.�������ڱ���ͬϵ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com