
��һ�ְ�ɫ��ĩ�����ܺ��� ��NaCl��
��NaCl�� ��
�� ��
�� �е�һ�ֻ��֣��������¼���ʵ�飺
�е�һ�ֻ��֣��������¼���ʵ�飺
��ȡ������ɫ��ĩ��������������ˮ�����а�ɫ�������ɣ���Һ��ɫ��
�������������еμ�ϡ���ᣬ�����ݲ����������μ�ϡ���ᣬ����������ɫ��������ڣ�
�˰�ɫ��ĩ�У��϶����ڵ�������(�ѧʽ����ͬ)__________�����ܴ��ڵ�������______________���϶������ڵ�������_________________��
д�����������е����ӷ���ʽ��
��_________________________________________��
��_________________________________________��
 ����ѵ��ϵ�д�
����ѵ��ϵ�д� ��ĩ�����ϵ�д�
��ĩ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
- 3 |
2- 4 |
2- 3 |
- 3 |
- 4 |
+ 4 |
| ʵ����� | ���� |
| a��ȡ������ĩ����ˮ���� | ȫ���ܽ⡢��Һ��ɫ�� |
| b����������Һ����������NaOH��Һ������ | ���������� |
| ʵ����� | ���� |
| c��ȡ������ĩ�������� | ���������� |
| b��ȡ������ĩ����ϡBaCl2��ϡ����Ļ��Һ | �а�ɫ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�058
��һ�ְ�ɫ��ĩ�����ܺ��� �е�һ�ֻ��֣��������¼���ʵ�飺
�е�һ�ֻ��֣��������¼���ʵ�飺
��ȡ������ɫ��ĩ��������������ˮ�����а�ɫ�������ɣ���Һ��ɫ��
�������������еμ�ϡ���ᣬ�����ݲ����������μ�ϡ���ᣬ����������ɫ��������ڣ��˰�ɫ��ĩ�У��϶�����������(�ѧʽ����ͬ)________________________________________�����ܴ��ڵ�������___________________________________________���϶������ڵ�������____________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������������ ���ͣ�058
��һ�ְ�ɫ��ĩ�����ܺ��� ��NaCl��
��NaCl�� ��
�� ��
�� ��һ�ֻ��֣��������¼���ʵ�飺
��һ�ֻ��֣��������¼���ʵ�飺
��ȡ������ɫ��ĩ��������������ˮ�����а�ɫ�������ɣ���Һ��ɫ��
�������������еμ�ϡ���ᣬ�����ݲ����������μ�ϡ���ᣬ����������ɫ��������ڣ�
�˰�ɫ��ĩ�У��϶����ڵ�������(�ѧʽ����ͬ)________________�����ܴ��ڵ�������_____________���϶������ڵ�������________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
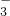 ��SO
��SO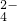 ��CO
��CO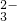 ��HCO
��HCO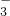 ��MnO
��MnO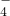 �������ӣ�Na+��Mg2+��Al3+��Ba2+��Fe2+��Fe3+��Cu2+��NH
�������ӣ�Na+��Mg2+��Al3+��Ba2+��Fe2+��Fe3+��Cu2+��NH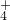 ���ֽ��ð�ɫ��ĩ��������ʵ�飺
���ֽ��ð�ɫ��ĩ��������ʵ�飺| ʵ����� | ���� |
| a��ȡ������ĩ����ˮ���� | ȫ���ܽ⡢��Һ��ɫ�� |
| b����������Һ����������NaOH��Һ������ | ���������� |
| ʵ����� | ���� |
| c��ȡ������ĩ�������� | ���������� |
| b��ȡ������ĩ����ϡBaCl2��ϡ����Ļ��Һ | �а�ɫ�������� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com