
������16�֣�
(1)ij������ṹ��ʽ��ͼ���������ʾ�Ļ�����ش����⣺
�û������У������Ţٵ�������________�������Ţߵ�������________���û���������________�������������ˮ�γɵģ�д���û�����ˮ�����ɵİ�����Ľṹ��ʽ(��дһ��)��________����д���˰�����������������Һ��Ӧ�Ļ�ѧ����ʽ��_________________
(2)ij�л��ﺬ��C��N��H��O����Ԫ�أ���ͼΪ���л�������ģ�͡�
�ٸ��л���Ļ�ѧʽ______________________________��
�ṹ��ʽ______________________________________________________��
�ڸ��л�����ܷ����Ļ�ѧ��Ӧ��(����)________��
a��ˮ�⡡b���Ӿ�c��ȡ�� d����ȥe������
�۸��л����ˮ�ⷴӦ�Ļ�ѧ����ʽ__________________________________��
(1)�������Ȼ���4��H2N��CH2��COOH
H2N��CH2��COOH��NaOHϡH2SO4H2N��CH2��COONa��H2O
(���������ְ����ἰ��Ӧ�Ļ�ѧ����ʽ)
(2)��C5H11NO2��CH3CH2CONHCH2CH2OH����acde
��CH3CH2CONHCH2CH2OH��H2OCH3CH2COOH��NH2CH2CH2OH
����:(1)�������γ��ļ�ʱ�ǰ������⣬�Ȼ���ȥ�ǻ����Ȼ���������Ӧ��
(2)���ݸ�ԭ�ӵijɼ��ص��֪��C��N��H��O�ֱ��γ�4��3��1��2�����ۼ����ɴ˿ɵõ����л���Ļ�ѧʽ�ͽṹ��ʽ���÷����к����ļ����ǻ���������֪�����ʡ�


 ���ɶ��ȫ���ƿؾ�ϵ�д�
���ɶ��ȫ���ƿؾ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������16�֣����ᱵ��Ψһ�����ı��Σ���ҵ�������ᱵ��Ϊԭ��ͨ���������̷�Ӧ�����Ʊ�п���ף�BaSO4+ZnS���������⡣��𩷯ΪZnSO4•7H2O��
��1�����������й���7����ѧ��Ӧ��������____________������������ԭ��Ӧ��
��2��д���������������C�ĵ���ʽ��____________________��_______________��
��3��д��F��G�Ļ�ѧʽ�� F_____________��G_________________��
��4��д�����л�ѧ��Ӧ����ʽ��
��Ӧ��__________________________________________________________��
��Ӧ��____________________________________________________��
��5��ȡп������16.5g����100mL 1mol/L��H2SO4 ��Һ�У��ų�H2S ����1008mL��������ɱ�״����
�ٲ�����Һ����仯��������Һ������������ʵ���Ũ��Ϊ________mol/L
�ڼ���������Һ��H2S ��Ϊʹп���Ӹպ���ȫ������Ӧ���� 1 mol/L��NaOH��Һ_____mL
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��㶫ʡ���и߶���ѧ�������ʼ����ۻ�ѧ�Ծ����������� ���ͣ������
����16�֣�������������ͼ�ǽṹ��ʽΪCH3CH2CH2OH��CH3CH(OH)CH3�������л��������1H�˴Ź�����ͼ�����ж���һ����CH3CH(OH)CH3��1H��NMR��ͼ����˵�����ɡ���6�֣�
�ڡ�0.2 mol�л����0.4 mol O2���ܱ�������ȼ�պ�IJ���ΪCO2��CO��H2O��g����ȼ�պ����Щ���ᆳ��ŨH2SO4����������10.8 g����ͨ�����ȵ�CuO��ַ�Ӧ������������3.2 g�����������ͨ����ʯ�ұ���ȫ���գ���������17.6 g���������õ������ԭ������H-1��C-12��O-16��Cu-64��
��1���ƶϸ��л���ķ���ʽ����6�֣�
��2����0.2 mol���л�����������Ľ�������ȫ��Ӧ��ų�4.48 L H2����״��������ȷ���л���Ľṹ��ʽ����4�֣�?
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ����һ�и߶���ѧ�����п��Ի�ѧ�������Ծ� ���ͣ������
������16�֣�
(1)ij������ṹ��ʽ��ͼ���������ʾ�Ļ�����ش����⣺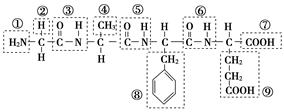
�û������У������Ţٵ�������________�������Ţߵ�������________���û���������________�������������ˮ�γɵģ�д���û�����ˮ�����ɵİ�����Ľṹ��ʽ(��дһ��)��________����д���˰�����������������Һ��Ӧ�Ļ�ѧ����ʽ��_________________
(2)ij�л��ﺬ��C��N��H��O����Ԫ�أ���ͼΪ���л�������ģ�͡�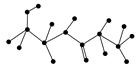
�ٸ��л���Ļ�ѧʽ______________________________��
�ṹ��ʽ_______________________________________________ _______��
_______��
�ڸ��л�����ܷ����Ļ�ѧ��Ӧ��(����)________��
a��ˮ�⡡b���Ӿ�c��ȡ�� d����ȥe������
�۸��л����ˮ�ⷴӦ�Ļ�ѧ����ʽ__________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���㽭ʡ�߶���ѧ�����п��Ի�ѧ�������Ծ� ���ͣ������
������16�֣�
(1)ij������ṹ��ʽ��ͼ���������ʾ�Ļ�����ش����⣺
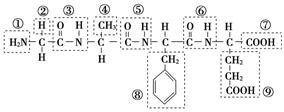
�û������У������Ţٵ�������________�������Ţߵ�������________���û���������________�������������ˮ�γɵģ�д���û�����ˮ�����ɵİ�����Ľṹ��ʽ(��дһ��)��________����д���˰�����������������Һ��Ӧ�Ļ�ѧ����ʽ��_________________
(2)ij�л��ﺬ��C��N��H��O����Ԫ�أ���ͼΪ���л�������ģ�͡�
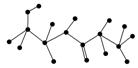
�ٸ��л���Ļ�ѧʽ______________________________��
�ṹ��ʽ______________________________________________________��
�ڸ��л�����ܷ����Ļ�ѧ��Ӧ��(����)________��
a��ˮ�⡡b���Ӿ�c��ȡ�� d����ȥe������
�۸��л����ˮ�ⷴӦ�Ļ�ѧ����ʽ__________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com