
ĻĀĶ¼ŹĒA”¢B”¢C”¢D”¢E”¢FµČ¼øÖÖ³£¼ūÓŠ»śĪļÖ®¼äµÄ×Ŗ»Æ¹ŲĻµĶ¼”£ĘäÖŠAŹĒĆę·ŪµÄÖ÷ŅŖ³É·Ö£»CŗĶE·“Ó¦ÄÜÉś³ÉF£¬F¾ßÓŠĻćĪ¶”£BÓėEµÄŹµŃéŹ½ĻąĶ¬
![]()
ŌŚÓŠ»śĪļÖŠ£¬·²ŹĒ¾ßÓŠ-CHO½į¹¹µÄĪļÖŹ£¬¾ßÓŠČēĻĀŠŌÖŹ£ŗ
£Ø1£©ÓėŠĀÖʵÄĒāŃõ»ÆĶŠü×ĒŅŗ·“Ó¦£¬²śÉś×©ŗģÉ«µÄ³Įµķ£¬
£Ø2£©ŌŚ“߻ƼĮµÄ×÷ÓĆĻĀ£¬-CHO±»ŃõĘųŃõ»ÆĪŖ-COOH,¼“£ŗ
2R-CHO+O2![]() 2R-COOH
2R-COOH
øł¾Żøł¾ŻŅŌÉĻŠÅĻ¢¼°ø÷ĪļÖŹµÄ×Ŗ»Æ¹ŲĻµĶź³ÉĻĀĮŠø÷Ģā£ŗ
(1) AµÄ»ÆѧŹ½ĪŖ £¬BµÄ½į¹¹¼ņŹ½ĪŖ”””””””””” ”””””””£ÓėB»¤ĪĄĶ¬·ÖŅģ¹¹ĢåµÄŅ»ÖÖĪļÖŹµÄĆū³Ę ”£
(2) FŌŚĻ”ĮņĖįÖŠ·¢ÉśĖ®½ā·“Ó¦µÄ·“Ó¦·½³ĢŹ½
(3) EÓė°±Ė®·¢ÉśµÄĄė×Ó·½³ĢŹ½
EÓėŠ”ĖÕ“ņČÜŅŗ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ”””””””””””””””””””””””””” ””””
(4) ĘäÖŠÄÜÓėŠĀÖĘĒāŃõ»ÆĶŠü×ĒŅŗ²śÉś×©ŗģÉ«µÄ³ĮµķµÄĪļÖŹÓŠ £ØĢīĆū³Ę£©”£
(5) Š“³öÄĘÓėCµÄ·“Ó¦·½³ĢŹ½
(6) Š“³öC”śDµÄ·“Ó¦·½³ĢŹ½
(7) C+E”śFµÄ·“Ó¦·½³ĢŹ½
”¾“š°ø”æ£Ø1£©£ØC6H10O5£©£ī CH2OH£ØCHOH£©4CHO ¹ūĢĒ
£Ø2£©CH3COOC2H5+H2O![]() CH3COOH+C2H5OH
CH3COOH+C2H5OH
£Ø3£©CH3COOH+NH3.H2O£½CH3COO-+NH4++H2O
CH3COOH+NaHCO3£½CH3COONa+CO2”ü+H2O
£Ø4£©BE
(5)2CH3CH2OH+2Na”ś2CH3CH2ONa+H2”ü
(6) 2CH3CH2OH+O2![]() 2CH3CHO+2H2O
2CH3CHO+2H2O
(7) C2H5OH+CH3COOH![]() CH3COOC2H5+H2O
CH3COOC2H5+H2O
”¾½āĪö”æøĆĶʶĻĢāµÄĶ»ĘĘæŚŌŚ”°Ćę·Ū”±ŗĶ”°BÓėEµÄŹµŃéŹ½ĻąĶ¬”±”£Ćę·ŪµÄÖ÷ŅŖ³É·ÖŹĒµķ·Ū”£ĶعżĢāÄæĢįŹ¾æÉĶĘÖŖAĪŖµķ·Ū£¬ÓÉÓŚA³ä·ÖĖ®½ā£¬ĖłŅŌĖ®½ā²śĪļĪŖµ„ĢĒ£¬¹ŹBĪŖĘĻĢŃĢĒ£»ĶعżCÉś³ÉDµÄĢõ¼žæÉÖŖCĪŖ“¼”¢DĪŖČ©£¬½įŗĻ¾ĘµÄÄšÖĘ£¬æÉÖŖC”¢D·Ö±šĪŖŅŅ“¼”¢ŅŅČ©£¬¹ŹEĪŖŅŅĖį£»ÓÖŅņĪŖC”¢EÉś³ÉF£¬¹ŹFĪŖŅŅĖįŅŅõ„£¬¹ŹFŌŚĻ”ĮņĖįÖŠµÄĖ®½ā·“Ó¦¾ĶŹĒŅŅĖįŅŅõ„µÄĖ®½ā”£Č»ŗóøł¾ŻĢāÄæŅŖĒó×÷³ö“š°ø”£


 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
£Ø16·Ö£©
AŹĒĆę·ŪÖŠµÄÖ÷ŅŖ³É·Ö£¬CÓėE·“Ó¦æÉÉś³ÉF£¬DÄÜÓėŠĀÖʵÄCu(OH)2Šü×ĒŅŗ·“Ó¦²śÉś×©ŗģÉ«³Įµķ”£ĻĀĶ¼ŹĒA”¢B”¢C”¢D”¢E”¢FµČ¼øÖÖ³£¼ūÓŠ»śĪļÖ®¼äµÄ×Ŗ»Æ¹ŲĻµĶ¼£ŗ
øł¾ŻŅŌÉĻŠÅĻ¢Ķź³ÉĻĀĮŠø÷Ģā£ŗ
£Ø1£©AµÄ»ÆѧŹ½ĪŖ____________£¬BµÄĆū³ĘĪŖ_____________”£
£Ø2£©CµÄ½į¹¹¼ņŹ½ĪŖ__________£¬DµÄ½į¹¹¼ņŹ½ĪŖ__________£¬FµÄ½į¹¹¼ņŹ½ĪŖ__________”£
£Ø3£©CŗĶE·“Ӧɜ³ÉFµÄ»Æѧ·½³ĢŹ½ĪŖ_________________________________”£
£Ø4£©EÓėŠ”ĖÕ“ņČÜŅŗ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ_______________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010Äź¹ć¶«Ź”Õ潶žÖŠøßŅ»µŚ¶žŃ§ĘŚĘŚÄ©æ¼ŹŌ£ØĄķ×Ū£©»Æѧ²æ·Ö ĢāŠĶ£ŗĢīæÕĢā
£Ø16·Ö£©
AŹĒĆę·ŪÖŠµÄÖ÷ŅŖ³É·Ö£¬CÓėE·“Ó¦æÉÉś³ÉF£¬DÄÜÓėŠĀÖʵÄCu(OH)2Šü×ĒŅŗ·“Ó¦²śÉś×©ŗģÉ«³Įµķ”£ĻĀĶ¼ŹĒA”¢B”¢C”¢D”¢E”¢FµČ¼øÖÖ³£¼ūÓŠ»śĪļÖ®¼äµÄ×Ŗ»Æ¹ŲĻµĶ¼£ŗ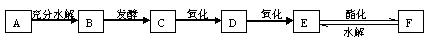
øł¾ŻŅŌÉĻŠÅĻ¢Ķź³ÉĻĀĮŠø÷Ģā£ŗ
£Ø1£©AµÄ»ÆѧŹ½ĪŖ____________£¬BµÄĆū³ĘĪŖ_____________”£
£Ø2£©CµÄ½į¹¹¼ņŹ½ĪŖ__________£¬DµÄ½į¹¹¼ņŹ½ĪŖ__________£¬FµÄ½į¹¹¼ņŹ½ĪŖ__________”£
£Ø3£©CŗĶE·“Ӧɜ³ÉFµÄ»Æѧ·½³ĢŹ½ĪŖ_________________________________”£
£Ø4£©EÓėŠ”ĖÕ“ņČÜŅŗ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ_______________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010Äź¹ć¶«Ź”øßŅ»µŚ¶žŃ§ĘŚĘŚÄ©æ¼ŹŌ£ØĄķ×Ū£©»Æѧ²æ·Ö ĢāŠĶ£ŗĢīæÕĢā
£Ø16·Ö£©
AŹĒĆę·ŪÖŠµÄÖ÷ŅŖ³É·Ö£¬CÓėE·“Ó¦æÉÉś³ÉF£¬DÄÜÓėŠĀÖʵÄCu(OH)2Šü×ĒŅŗ·“Ó¦²śÉś×©ŗģÉ«³Įµķ”£ĻĀĶ¼ŹĒA”¢B”¢C”¢D”¢E”¢FµČ¼øÖÖ³£¼ūÓŠ»śĪļÖ®¼äµÄ×Ŗ»Æ¹ŲĻµĶ¼£ŗ
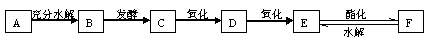
øł¾ŻŅŌÉĻŠÅĻ¢Ķź³ÉĻĀĮŠø÷Ģā£ŗ
£Ø1£©AµÄ»ÆѧŹ½ĪŖ____________£¬BµÄĆū³ĘĪŖ_____________”£
£Ø2£©CµÄ½į¹¹¼ņŹ½ĪŖ__________£¬DµÄ½į¹¹¼ņŹ½ĪŖ__________£¬FµÄ½į¹¹¼ņŹ½ĪŖ__________”£
£Ø3£©CŗĶE·“Ӧɜ³ÉFµÄ»Æѧ·½³ĢŹ½ĪŖ_________________________________”£
£Ø4£©EÓėŠ”ĖÕ“ņČÜŅŗ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ_______________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ¼ŖĮÖŹ”¼ŖĮÖŹŠĘÕĶØ֊ѧ2010-2011ѧğøßÖŠ±ĻŅµ°ąĆžµ× ĢāŠĶ£ŗĶʶĻĢā
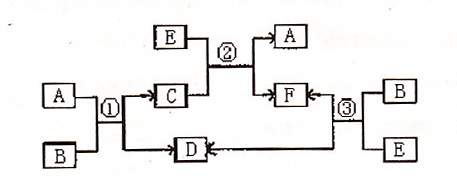 ĻĀĶ¼ŹĒA”¢B”¢C”¢D”¢E”¢FĮłÖÖĪļÖŹµÄ×Ŗ»Æ¹ŲĻµ”£ĘäÖŠBŹĒŅ»ÖÖ³£¼ūµÄĪŽÉ«ĪŽĪ¶µÄŅŗĢ壬CŹĒŅ»ÖÖÓŠ“ÅŠŌµÄ»ÆŗĻĪļ£¬EŹĒŅ»ÖÖĪŽÉ«ĪŽĪ¶µÄÓŠ¶¾ĘųĢ唣
ĻĀĶ¼ŹĒA”¢B”¢C”¢D”¢E”¢FĮłÖÖĪļÖŹµÄ×Ŗ»Æ¹ŲĻµ”£ĘäÖŠBŹĒŅ»ÖÖ³£¼ūµÄĪŽÉ«ĪŽĪ¶µÄŅŗĢ壬CŹĒŅ»ÖÖÓŠ“ÅŠŌµÄ»ÆŗĻĪļ£¬EŹĒŅ»ÖÖĪŽÉ«ĪŽĪ¶µÄÓŠ¶¾ĘųĢ唣
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©Š“³öCµÄĖ×Ćū£ŗ ”£
£Ø2£©Š“³öBµÄµē×ÓŹ½£ŗ ”£
£Ø3£©ŌŚB”¢D”¢E”¢FĖÄÖÖ·Ö×ÓÖŠ£¬ŗ¬ÓŠ·Ē¼«ŠŌ¼üµÄŹĒ£ØĢī»ÆѧŹ½£© ”£
£Ø4£©·“Ó¦¢Ś¢Ū¶¼ŹĒ¹¤ŅµÉĻ·Ē³£ÖŲŅŖµÄ·“Ó¦£¬ĒėŠ“³öÕāĮ½øö·“Ó¦µÄ»Æѧ·½³ĢŹ½”£
¢Ś £»
¢Ū £®
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com