
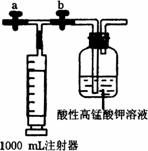
(1)̽��װ����ͼ��ʾ(a��bΪ���أ��г֡��̶�װ����ȥ)��
(2)̽��ԭ����
_________![]() +_________HCHO+_________H+
+_________HCHO+_________H+![]() _________Mn2++
_________Mn2++
_________CO2��+_________H2O
�뽫��ƽ�Ļ�ѧ������������Ӧ�ĺ����ϡ�
(3)ʵ�鲽�裺
�ټ��װ�õ������ԣ�������ǣ��ر�a��b�������ܽ�ע�����������³�ȡ���ٹر�a����b����ע�����������³�ȡ��������____________����ʱ����װ�����������á�
����__________(��д��������)ȷ��ȡ1.00 mL 1.0��10-4 mol��L-1��KMnO4��Һ�ڹ��ƿ�У�������2��3��2 mol��L-1�������ữ��
�۹ر�b����a����ע������ȡ1 000 mL��װ�ľ����ڵĿ������ر�a����b���ٻ������ƶ�ע����������������ȫ���������Ը��������Һ�С��������ƶ�ע����������ԭ����______________��
�����ظ�������9�Σ����Ը��������Һ�պ���Ϊ��ɫ(�����ȩ���������)�������ʱ���ڿ����м�ȩ��Ũ��Ϊ___________mg��m-3�����жϴ�ʱ����____________(��ܡ����ܡ�)��ס��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 �����¼�ȩ��һ����ɫ��������̼�����ζ�����壬������ˮ����ȷ�����°����»�����֮һ���ҹ��涨�����ҿ����м�ȩ���������Ũ��Ϊ0.08mg/m3��ijͬѧ��Ʋⶨ��ȩ�ķ������£������������������ԭ�����壩��
�����¼�ȩ��һ����ɫ��������̼�����ζ�����壬������ˮ����ȷ�����°����»�����֮һ���ҹ��涨�����ҿ����м�ȩ���������Ũ��Ϊ0.08mg/m3��ijͬѧ��Ʋⶨ��ȩ�ķ������£������������������ԭ�����壩���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
ij�о���ѧϰС����������·����ⶨij���ҿ����м�ȩ�ĺ����������������������ԭ�����壩��
��.�ⶨԭ����KMnO4��H+����ҺΪǿ����������������ȩ�Ͳ��ᣬ�䷴Ӧ�����ӷ���ʽΪ��
4![]() +5HCHO+12H+====4Mn2++5CO2��+11H2O
+5HCHO+12H+====4Mn2++5CO2��+11H2O
2![]() +5H2C2O4+6H+====2Mn2++10CO2��+8H2O
+5H2C2O4+6H+====2Mn2++10CO2��+8H2O
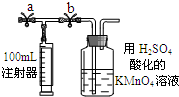
��.�ⶨװ�ã�����װ������ͼ��ʾ��a��bΪֹˮ�У�

��.ʵ�鲽�裺
�ټ��װ�������ԣ����������ã���
����A����ȷ��ȡ25.00 mL 1.00��10-3 mol��L-1�ĸ��������Һ���������ڹ��ƿ�в�����3��6 mol��L-1 H2SO4��Һ���á�
�۽�2.00��10-3 mol��L-1�IJ������Һ����A�����б��á�
�ܴ�a���ر�b����ע������ȡ100 mL��װ�����ڿ������ر�a����b���ٻ����ƶ�ע������������ȫ������������������Һ�У�ʹ���ַ�Ӧ��������ظ�4�Σ���5�Σ���
�ݽ����ƿ�е���Һת����ƿ�У���ϴ���ƿ2��3�Σ�����ϴ��Һȫ��ת����ƿ����
���ñ�������Һ�ζ���ƿ�е���Һ����¼�ζ������ĵIJ�����Һ�����
�����ظ�ʵ��2�Ρ�
��.���ݴ������ֱ���װ���깤��ĵ�1�졢��7�졢��30�죨����ʼ�ձ���ͨ�绻��״���������ڿ�������ȡ����ͨ��ʵ���������������ݣ�ÿ��ʵ����ȡ��KMnO4��Һ��Ϊ25.00 mL����
װ���n�� | ������Һ/mL | ��ȩ��Ũ��/mg��m-3 | |||
1 | 2 | 3 | ƽ��ֵ | ||
1 | 15.86 | 15.72 | 15.67 | 15.75 |
|
7 | 26.24 | 26.36 | 26.31 | 26.30 |
|
30 | 30.90 | 30.67 | 30.81 | 30.79 |
|
������������⣺
��1��A����������_________________ ��
��2����������ѹ������ʱ�ٶȹ��죬���ܻ����ʲô���������
_____________________________________________________________________��
��3����������û����ϴ�������ȩ�ĺ�����____________���ƫ�ߡ���ƫ�͡�����Ӱ�족����
��4����ʵ���Ƿ���Ҫָʾ���������Ҫ����д��ָʾ�������ƣ��������Ҫ����˵���յ�ʱ��ʵ������___________________________________________________________________��
��5������KMnO4��Һ�����ʵ���Ũ��Ϊc1 mol��L-1����ȡKMnO4��Һ�����ΪV1 mL��������Һ�����ʵ���Ũ��Ϊc2 mol��L-1�����IJ�����Һ��ƽ�����ΪV2 mL��
�������ڿ����м�ȩŨ�ȣ�mg��m-3���Ĵ���ʽΪ____________����30�����ڿ����м�ȩŨ��Ϊ____________�����жϴ�ʱ����____________����ܡ�����ס��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�����¼�ȩ��һ����ɫ��������̼�����ζ�����壬������ˮ��������������֯��WHO��ȷ�����°�����»�����֮һ���ҹ��涨�����ҿ����м�ȩ���������Ũ��Ϊ0.08 mg/m3��
ij�о���ѧϰС����������·����ⶨij���ҿ����м�ȩ�ĺ����������������������ԭ�����壩��
��1���ⶨԭ����KMnO4��H+����ҺΪǿ����������������ȩ�Ͳ��ᡣ
��Ӧ�����ӷ���ʽΪ��![]()
![]()
��2���ⶨװ�ã�����װ������ͼ��ʾ��a��bΪֹˮ�У���

��3��ʵ�鲽�裺
�ټ��װ�������ԣ����������ã���
����__________�����������ƣ�ȷ��ȡ25.00 mL 1.00��10-3 mol��L-1 �ĸ��������Һ���������ڹ��ƿ�в�����3��6 mol��L-1 H2SO4��Һ���á�
�۽�2.00��10-3 mol��L-1�IJ������Һ����__________�����������ƣ��б��á�
�ܴ�a���ر�b����ע������ȡ100 mL��װ�����ڿ������ر�a����b���ٻ����ƶ�ע������������ȫ���������Ը��������Һ�У�ʹ���ַ�Ӧ��������ظ�4�Σ���5�Σ������ѹ������ʱ�ٶȹ��죬���ܻ����ʲô���������______________________________��
�ݽ����ƿ�е���Һת����ƿ�У���ϴ���ƿ2��3�Σ�����ϴ��Һȫ��ת����ƿ�������û����ϴ�������ȩ�ĺ�����__________���ƫ�ߡ���ƫ�͡�����Ӱ�족����
���ò������Һ�ζ���ƿ�е���Һ������¼�ζ������ĵIJ�����Һ�����
��ʵ���Ƿ���Ҫָʾ�����������Ҫ����д��ָʾ�������ƣ��������Ҫ����˵���յ�ʱ��ʵ������______________________________��
�����ظ�ʵ�����Ρ�
��4�����ݴ������ֱ���װ���깤��ĵ�1�졢��7�졢��30�죨����ʼ�ձ���ͨ�绻��״���������ڿ�������ȡ����ͨ��ʵ���������������ݣ�ÿ��ʵ����ȡ��KMnO4��Һ��Ϊ25.00 mL����
| װ�ú� ��n�� | ������Һ/mL | ��ȩ��Ũ��mg/m3 | |||
| 1 | 2 | 3 | ƽ��ֵ | ||
| 1 | 15.86 | 15.72 | 15.67 | 15.75 | |
| 7 | 26.17 | 26.36 | 26.38 | 26.30 | |
| 30 | 30.90 | 30.67 | 30.81 | 30.79 | |
��KMnO4��Һ�����ʵ���Ũ��Ϊc1 mol��L-1��KMnO4��Һ�����ΪV1 mL��������Һ�����ʵ���Ũ��Ϊc2 mol��L-1��������Һ��ƽ�����ΪV2 mL��
�����ڿ����м�ȩŨ�ȣ�mg/m3���Ĵ���ʽΪ__________����30�����ڿ����м�ȩŨ��Ϊ__________����ȷ��С�������λ�������жϴ�ʱ����__________����ܡ����ܡ�����ס��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
��ȩ��Һ���û�����ֲ������ϲ�Ϊ��ɫ��״Һ�壬�²�Ϊˮ��Һ������ �����ϲ�����Ϊ��ȩ�ľۺ���(CH2O)![]() ���÷�������ȩ�����ҷе��ˮ�ߡ���֪��(CH2O)n��nCH2O(���Ի�����)
���÷�������ȩ�����ҷе��ˮ�ߡ���֪��(CH2O)n��nCH2O(���Ի�����)
(1)��ȩ��Һ���ڿ������ױ�������֤����ȩ�ѱ�������ʵ�������������
��
(2)��������ͼa��ʾװ����ȡ��ȩ����ƿװ(CH2O)n��6mol��Lϡ������Һ�����������ڣ�(CH2O)n�ֽ⣬�����ļ�ȩ���屻��ƿ�е�����ˮ���գ��������ܵĽ�ˮ����Ϊ (�a����b��)�����������л�����Һ���� ��������δ������ƿҺ���µ�ԭ���� ��
�����¼�ȩ�ж������°�����»�����֮һ���ҹ��涨�����ҿ����еļ�ȩ���������Ũ��Ϊ0.08mg��m3��ij��ѧ�о�С�������·����ⶨ�����еļ�ȩ��Ũ��(���������������ԭ������)��
�ⶨԭ�������Ը�����ؿ�������ȩ�Ͳ��ᣬ�����ӷ���ʽΪ��
4MnO![]() +5HCHO+12H+==4Mn2++5CO2��+11H2O
+5HCHO+12H+==4Mn2++5CO2��+11H2O
( )MnO![]() +( )H2C2O4+( )H+��( )Mn2++( )CO2��+( )H2O
+( )H2C2O4+( )H+��( )Mn2++( )CO2��+( )H2O
�ⶨװ����ͼb��ʾ��
ʵ�鲽�裺
(3)���װ�õ������ԣ���μ��װ�õ�������?
��
(4)�� (����������)ȡ�������Ը��������Һװ����ƿ�С�
(5)��a���ر�b����ע������ȡ100 mL��װ�����ڵĿ������ر�a����b���ٻ����ƶ�ע������������ȫ���������Ը��������Һ�У�ʹ���ַ�Ӧ��������ظ���Σ����ѹ��������ٶȹ��죬�Բⶨ������Ӱ��?
��

(6)�ò���ζ����ƿ�е���Һ��(�Ҫ����Ҫ��) ָʾ�����յ��ʵ�������� ����������ص�Ũ��Ϊc1�����ΪV1mL�������Ũ��Ϊc2�����IJ�����Һ��ƽ�����ΪV2 mL�����ȩ��Ũ���� mg��m3��(���ȡ�Ŀ������Ϊ100mL)
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com