
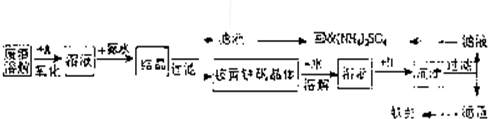
��ҵ����������������Fe2+��Fe3+�������μ�����CaO��MgO���Ʊ��ߵ���������(Fe2O3 )�ͻ���(NH4)2SO4�����������������£�
��1���ڷ����ܽ����ʱ��Ӧѡ��__________�ܽ⣨����ĸ����
A����ˮ B���������� C������ D������
��2������A��һ������������ҵ�����ѡ�� ����ѡ��ʹ�õ��У�������Cl2��MnO2������������ ��
��3��������ͼ�й����ݣ�����Ϊ��ҵ����������ʱӦ���Ƶ������ǣ� ��
��4�����ᾧ����Ӧ�Ļ�ѧ����ʽΪ_________________ ____________________��
��5���������顰��Һ���к���NH4����ʵ�鷽���� ��
��1��D�� 2�� ����2��������2�֣��� ԭ����Դ���ף��ɱ��ͣ���������Ⱦ�����������ʡ���2�֣����������㼴�ɣ�
��3����Һ�¶ȿ�����80�棨1�֣���pH������1.5��1�֣�������ʱ��Ϊ4Сʱ���ң�2�֣�
��4��3Fe2(SO4)3 �� 2NH3��H2O �� 10H2O�� (NH4)2Fe6(SO4)4(OH)12��+5H2SO4 ��3�֣�
��5��ȡ������Һ���Թ��У����Թ��м���������Ũ����������Һ�����ȡ���ʪ��ĺ�ɫʯ����ֽ�����Թܿڴ�����ɫʯ����ֽ����ɫ��˵����Һ�к���NH4+����3�֣�
����:��


 ��Ȥ����¹�֪��ϵ�д�
��Ȥ����¹�֪��ϵ�д� Ӣ��СӢ������Ĭдϵ�д�
Ӣ��СӢ������Ĭдϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�콭��ʡ̩����ѧ������ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ������
��12�֣���ҵ����������������Fe2+��Fe3+�������μ�����CaO��MgO���Ʊ��ߵ��������죨Fe2O3���ͻ���(NH4)2SO4�����������������£�
��1���ڷ����ܽ�ʱ��Ҫʹ���ᣬӦѡ��__________��Ϊ����߷����Ľ�ȡ�ʣ��ɲ��õĴ�ʩ����Щ��
_____________________________________________________________������д�����㣩��
��2������A��һ������������ҵ�����ѡ��_____________����ѡ��ʹ�õ��У�������Cl2��MnO2������������ ������д�����㣩��
��3�����ᾧ����Ӧ�Ļ�ѧ����ʽΪ__________________________________________________��
��4���������������жദ�����˹��˲�����ʵ�����������Ӧ�IJ�����Ҫ�õ��IJ����������ձ���____________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�긣��ʡ�����ڶ���ģ�⿼�ԣ����ۣ���ѧ���� ���ͣ������
��ҵ����������������Fe2+��Fe3+�������μ�����CaO��MgO���Ʊ��ߵ���������(Fe2O3 )�ͻ���(NH4)2SO4�����������������£�
��1���ڷ����ܽ����ʱ��Ӧѡ��__________�ܽ⣨����ĸ����
A����ˮ B���������� C������ D������
��2������A��һ������������ҵ�����ѡ�� ����ѡ��ʹ�õ��У�������Cl2��MnO2������������ ��
��3��������ͼ�й����ݣ�����Ϊ��ҵ����������ʱӦ���Ƶ������ǣ�
��

��4�����ᾧ����Ӧ�Ļ�ѧ����ʽΪ
��
��5���������顰��Һ���к���NH4����ʵ�鷽����
��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com