
ФГЩеМюШмвКжаКЌгаЩйСПдгжЪ(ВЛгыбЮЫсЗДгІ)ЃЌЯжгУжаКЭЕЮЖЈВтЖЈЦфХЈЖШЁЃ
ЃЈ1ЃЉЕЮЖЈЃКЂйгУ ЪНЕЮЖЈЙмЪЂзАc mol/LбЮЫсБъзМвКЁЃШчЭМБэЪОФГДЮЕЮЖЈЪБ50 mLЕЮЖЈЙмжаЧАКѓвКУцЕФЮЛжУЁЃЧыНЋгУШЅЕФБъзМбЮЫсЕФЬхЛ§ЬюШыЂлБэПеИёжаЃЌДЫЪБЕЮЖЈЙмжавКЬхЕФЬхЛ§ mLЁЃ
ЂкЯТБэЪЧ4жжГЃМћжИЪОМСЕФБфЩЋЗЖЮЇЃК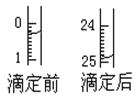
| жИЪОМС | ЪЏШя | МзЛљГШ | МзЛљКь | ЗгЬЊ |
| БфЩЋЗЖЮЇЃЈpHЃЉ | 5ЃЎ0ЁЊ8ЃЎ0 | 3ЃЎ1ЁЊ4ЃЎ4 | 4ЃЎ4ЁЊ6ЃЎ2 | 8ЃЎ2ЁЊ10ЃЎ0 |
| ЕЮЖЈађКХ | Д§ВтвКЬхЛ§(mL) | ЫљЯћКФбЮЫсБъзМвКЕФЬхЛ§(mL) | ||
| ЕЮЖЈЧА | ЕЮЖЈКѓ | ЯћКФЕФЬхЛ§ | ||
| 1 | V | 0ЃЎ50 | 25ЃЎ80 | 25ЃЎ30 |
| 2 | V | | | |
| 3 | V | 6ЃЎ00 | 31ЃЎ35 | 25ЃЎ35 |
ЃЈ1ЃЉЂйЫс 24ЃЎ60 Дѓгк25ЃЎ10 ЂкЗгЬЊ
ЃЈ2ЃЉ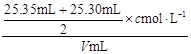
ЃЈ3ЃЉЮогАЯь ЦЋЕЭ ЦЋИп ЦЋЕЭ
НтЮіЪдЬтЗжЮіЃКЃЈ1ЃЉЫсадШмвКЛђбѕЛЏадЕФЮяжЪЕФШмвКгУЫсЪНЕЮЖЈЙмЃЛгУШЅЕФБъзМЫсШмвКЕФЬхЛ§ЮЊЃК24ЃЎ90Ѓ0ЃЎ30=24ЃЎ60mlЃЎгЩгкЪЙгУЕФЪЧ50 mLЕЮЖЈЙмЃЌЫљвдДЫЪБЕЮЖЈЙмжаДјгаПЬЖШЕФВПЗжШмвКЕФЬхЛ§ЮЊ50 mLЃ24ЃЎ90=25ЃЎ10mlЁЃдкЕЮЖЈЙмЯТБпЮоПЬЖШЕФВПЗжЛЙгавКЬхЃЌДЫЪБЕЮЖЈЙмжавКЬхЕФЬхЛ§Дѓгк25ЃЎ10mlЃЎ ЂкЪЏШябеЩЋБфЛЏВЛУїЯдЃЌЫљвдвЛАуВЛзіЫсМюЕЮЖЈЕФжИЪОМСЁЃгУЗгЬЊЛђМзЛљГШзїжИЪОМСЁЃгЩгкМзЛљГШЕФБфЩЋЗЖЮЇЪЧ3ЃЎ1ЁЊ4ЃЎ4ЃЌЗгЬЊЕФБфЩЋЗЖЮЇЪЧ8ЃЎ2ЁЊ10ЃЎ0ЁЃЮЊСЫМѕЩйЪЕбщЮѓВюЃЌвЊгУЗгЬЊЯрЖдИќОЋШЗЁЃЃЈ2ЃЉШ§ДЮЕЮЖЈЯћКФЕФБъзМHClЕФЬхЛ§ЗжБ№ЪЧ25ЃЎ30mlЁЂ24ЃЎ60mlЁЂ25ЃЎ35mlЁЃгЩгк24ЃЎ60mlЯрЖдЮѓВюНЯДѓЁЃЩсШЅЁЃвђДЫЯћКФЕФБъзМЫсШмвКЕФЬхЛ§ЮЊ(25ЃЎ30mlЃЋ25ЃЎ35ml)ЁТ2=25ЃЎ325mlЃЎЃЎИљОнЫсМюЧЁКУжаКЭЪБЕФЮяжЪЕФСПЯрЕШ ЃЌПЩЕУc(Мю)=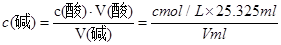 ЁЃЃЈ3ЃЉAЃЎШєЕЮЖЈЧАгУеєСѓЫЎГхЯДзЖаЮЦПЃЌгЩгкД§ВтЕФМюШмвКжаЕФШмжЪЕФжЪСПМАЮяжЪЕФСПУЛгаБфЛЏЃЌЫљвдЖдВтЖЈНсЙћЮогАЯьЁЃBЃЎЖСЪ§ЪБЃЌШєЕЮЖЈЧАбіЪгЃЌЖСЪ§ЦЋДѓЃЛЕЮЖЈКѓИЉЪгЃЌЖСЪ§ЦЋаЁЁЃдђЯћКФЕФБъзМЫсШмвКЕФЬхЛ§ЛсЦЋаЁЁЃвђДЫвдДЫЮЊБъзММЦЫуЕФД§ВтШмвКЕФХЈЖШОЭЦЋЕЭЁЃCЃЎШєдкЕЮЖЈЙ§ГЬжаВЛЩїНЋЪ§ЕЮЫсвКЕЮдкзЖаЮЦПЭтЃЌдђЯћКФЕФБъзМЫсШмвКЬхЛ§ЦЋДѓЃЌвдДЫЮЊБъзММЦЫуЕФД§ВтШмвКЕФХЈЖШОЭЦЋИпЁЃDЃЎЕЮМгбЮЫсЫйЖШЙ§ПьЃЌЮДГфЗжеёЕДЃЌИеПДЕНШмвКБфЩЋЃЌСЂПЬЭЃжЙЕЮЖЈЃЌдђЯћКФЕФБъзМЫсШмвКЕФЬхЛ§ЦЋЩйЃЌЛсЪЙВтЖЈНсЙћЦЋЕЭЁЃ
ЁЃЃЈ3ЃЉAЃЎШєЕЮЖЈЧАгУеєСѓЫЎГхЯДзЖаЮЦПЃЌгЩгкД§ВтЕФМюШмвКжаЕФШмжЪЕФжЪСПМАЮяжЪЕФСПУЛгаБфЛЏЃЌЫљвдЖдВтЖЈНсЙћЮогАЯьЁЃBЃЎЖСЪ§ЪБЃЌШєЕЮЖЈЧАбіЪгЃЌЖСЪ§ЦЋДѓЃЛЕЮЖЈКѓИЉЪгЃЌЖСЪ§ЦЋаЁЁЃдђЯћКФЕФБъзМЫсШмвКЕФЬхЛ§ЛсЦЋаЁЁЃвђДЫвдДЫЮЊБъзММЦЫуЕФД§ВтШмвКЕФХЈЖШОЭЦЋЕЭЁЃCЃЎШєдкЕЮЖЈЙ§ГЬжаВЛЩїНЋЪ§ЕЮЫсвКЕЮдкзЖаЮЦПЭтЃЌдђЯћКФЕФБъзМЫсШмвКЬхЛ§ЦЋДѓЃЌвдДЫЮЊБъзММЦЫуЕФД§ВтШмвКЕФХЈЖШОЭЦЋИпЁЃDЃЎЕЮМгбЮЫсЫйЖШЙ§ПьЃЌЮДГфЗжеёЕДЃЌИеПДЕНШмвКБфЩЋЃЌСЂПЬЭЃжЙЕЮЖЈЃЌдђЯћКФЕФБъзМЫсШмвКЕФЬхЛ§ЦЋЩйЃЌЛсЪЙВтЖЈНсЙћЦЋЕЭЁЃ
ПМЕуЃКПМВщЫсМюжаКЭЕЮЖЈЕФвЧЦїКЭжИЪОМСЕФбЁдёЁЂЪ§ОнЕФДІРэЁЂЮѓВюЕФЗжЮіМАХЈЖШЕФМЦЫуЕФжЊЪЖЁЃ


 аТЫМЮЌКЎМйзївЕЯЕСаД№АИ
аТЫМЮЌКЎМйзївЕЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃКЬюПеЬт
ПЦбаЁЂЩњВњжаГЃЩцМАФЦЁЂСђМАЦфЛЏКЯЮяЁЃ
ЃЈ1ЃЉЙЄвЕЩЯгУNa2CO3ШмвКДІРэЫЎЙИжаЕФCaSO4ЃЌЗДгІЕФРызгЗНГЬЪНЮЊ___________________ЃЛЪЕбщЪвжаЃЌNa2SШмвКГЄЦкЗХжУгаСђЮіГіЃЌдвђЮЊ___________________ЃЈгУРызгЗНГЬЪНБэЪОЃЉЁЃ
ЃЈ2ЃЉЯТЭМЪЧДѓаЭаюЕчЯЕЭГЕФЪОвтЭМЁЃзѓгвСНВрЮЊЕчНтжЪДЂЙоЃЌжабыЮЊЕчГиЃЌЗДгІдРэЮЊЃК ЃЌЕчНтжЪЭЈЙ§БУдкДЂЙоКЭЕчГиМфбЛЗЃЛРызгбЁдёадФЄжЛдЪаэФЦРызгЭЈЙ§ЁЃ
ЃЌЕчНтжЪЭЈЙ§БУдкДЂЙоКЭЕчГиМфбЛЗЃЛРызгбЁдёадФЄжЛдЪаэФЦРызгЭЈЙ§ЁЃ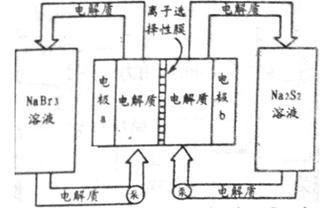
ЕБаюЕчГиЗХЕчЪБЃЌЕчГижаNa+ЕФвЦЖЏЗНЯђЪЧ_________ЃЈЬюЁАaЁњbЁБЛђЁАbЁњaЁБЃЉЃЌЕчМЋaЕФЕчМЋЗДгІЪНЮЊ_______________ЃЛЕБаюЕчГиДІгкГфЕчзДЬЌЪБЃЌЕчМЋbЕФЕчМЋЗДгІЪНЮЊ___________ЁЃгУИУЕчГизіЕчдДЃЌВЩгУЖшадЕчМЋЕчНт200 mL 1 molЁЄLЃ1ЕФAgNO3ШмвКЃЌЕБвѕМЋжЪСПдіМг2.16 gЪБЃЌЕчНтКѓШмвКЕФpHЮЊ_________ЃЈВЛПМТЧШмвКЬхЛ§БфЛЏЃЉЁЃ
ЃЈ3ЃЉЙЄвЕЩЯЃЌгУNa2SO3ШмвКзїЮЊЮќЪевКПЩЮќЪебЬЦјжаЕФSO2ЃЌЮќЪеSO2Й§ГЬжаЃЌШмвКpHгы ЙиЯЕШчЯТБэЃК
ЙиЯЕШчЯТБэЃК
 | 91ЁУ9 | 1ЁУ1 | 9ЁУ91 |
| pHЃЈ25ЁцЃЉ | 8.2 | 7.2 | 6.2 |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃКМЦЫуЬт
BaЃЈNO3ЃЉ2ПЩгУгкЩњВњТЬЩЋбЬЛЈЁЂТЬЩЋаХКХЕЏЁЂеЈвЉЁЂЬеДЩгдвЉЕШЁЃБЕбЮаавЕЩњВњжаХХГіДѓСПЕФБЕФр[жївЊКЌгаBaCO3ЁЂ BaSO3ЁЂ BaЃЈ FeO2ЃЉ2ЕШ]ЃЌФГжївЊЩњВњBaCO3ЁЂ BaSO4ЕФЛЏЙЄГЇРћгУБЕФржЦШЁBaЃЈNO3ЃЉ2ОЇЬхЃЈВЛКЌНсОЇЫЎЃЉЃЌЦфВПЗжЙЄвеСїГЬШчЯТЃК
гжвбжЊЃК
ЂйFe3+КЭFe2+вдЧтбѕЛЏЮяаЮЪНГСЕэЭъШЋЪБЃЌШмвКЕФpHЗжБ№ЮЊ3ЃЎ2КЭ9ЃЎ7ЃЛ
ЂкBaЃЈNO3ЃЉ2ОЇЬхЕФЗжНтЮТЖШЃК592ЁцЃЛ
ЂлKSPЃЈBaSO4ЃЉ=1ЃЎlxl0Ѓ10, KSPЃЈBaCO3ЃЉ=5ЃЎ1ЁС10Ѓ9ЃЎ
ЃЈ1ЃЉИУГЇЩњВњЕФBaCO3вђКЌгаЩйСПBaSO4ЖјВЛДПЃЌЬсДПЕФЗНЗЈЪЧЃКНЋВњЦЗМгШызуСПЕФБЅКЭNa2CO3ШмвКжаЃЌГфЗжНСАшЃЌЙ§ТЫЃЌЯДЕгЁЃЪдгУРызгЗНГЬЪНЫЕУїЬсДПдРэЃК______________ ЁЃ
ЃЈ2ЃЉЩЯЪіСїГЬЫсШмЪБЃЌBaЃЈFeO2ЃЉ2гыHNO3ЗДгІЩњГЩСНжжЯѕЫсбЮЃЌЗДгІЕФЛЏбЇЗНГЬЪНЮЊЃК
ЁЃ
ЃЈ3ЃЉИУГЇНсКЯБОГЇЪЕМЪЃЌбЁгУЕФXЮЊ ЃЈЬюађКХЃЉЃЛ
| AЃЎBaCl2 | BЃЎBaCO3 | CЃЎBaЃЈNO3ЃЉ2 | DЃЎBaЃЈOHЃЉ2 |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃКЪЕбщЬт
ЃЈ16ЗжЃЉNH3дкЩњЛюЩњВњжагУЭОЙуЗКЁЃ
ЃЈ1ЃЉЯТСаЙигкNH3ЛђАБЫЎЕФЪЕбщФмДяЕНФПЕФЕФЪЧ ЃЈЬюБрКХЃЉ
| БрКХ | A | B | C | D |
| ЪЕбщ зАжУ | 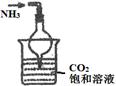 | 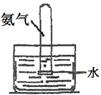 |  | 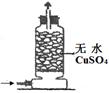 |
| ЪЕбщ ФПЕФ | ЪЕбщЪвФЃФтКюЪЯжЦМюЗЈжЦБИNH4HCO3 | бщжЄNH3взШмгкЫЎ | жЦБИвјАБШмвК | ИЩдяNH3 |

| ЪЕбщВНжш | ЪЕбщФПЕФ |
| ВНжш1ЃК ЂйгУМюЪНЕЮЖЈЙмШЁ20.00ml 0.1mol/L ЕФАБЫЎгкзЖаЮЦПжаЃЌМгШыМИЕЮ ЁЃ Ђк ЁЃ Ђл ЃЌЭЃжЙЕЮЖЈЃЌМЧТМЪ§ОнЁЃжиИДВтЖЈ2-3ДЮЁЃ | ВтЖЈАБЫЎЕФзМШЗХЈЖШЁЃ |
| ВНжш2ЃК ЃЛ | ЃЛ |
| ВНжш3ЃКЭЈЙ§МЦЫуЕУГіАБЫЎЕФЕчРыГЃЪ§ЁЃ | |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃКЪЕбщЬт
МзЁЂввСНЮЛЭЌбЇЩшМЦгУЪЕбщШЗЖЈФГЫсHAЪЧШѕЕчНтжЪЃЌДцдкЕчРыЦНКтЃЌЧвИФБфЬѕМўЦНКтЗЂЩњвЦЖЏЁЃЪЕбщЗНАИШчЯТЃК
МзЃКШЁДПЖШЯрЭЌЃЌжЪСПЁЂДѓаЁЯрЕШЕФаПСЃгкСНжЇЪдЙмжаЃЌЭЌЪБМгШы 0.1 molЁЄL-1ЕФHAШмвКЁЂЯЁбЮЫсИї10 mLЃЌАДЭМзАКУЃЌЙлВьЯжЯѓЁЃ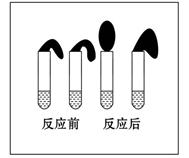
ввЃКЂй гУpHМЦВтЖЈХЈЖШОљЮЊ0.1 molЁЄL-1ЕФHAШмвККЭЯЁбЮЫсЕФpHЃЛ
Ђк дйШЁ0.1 molЁЄL-1ЕФHAШмвККЭЯЁбЮЫсИї2ЕЮ(1ЕЮдМЮЊ1/20 mL)ЗжБ№ЯЁЪЭжС100 mLЃЌдйгУpHМЦВтЦфpHБфЛЏЁЃ
(1)ввЕФЗНАИжаЫЕУїHAЪЧШѕЕчНтжЪЕФРэгЩЪЧЃКВтЕУ0.1 molЁЄL-1ЕФHAШмвКЕФ
pH 1(ЬюЁАЃОЁБЁЂЁАЃМЁБЛђЁА=ЁБ)ЃЛМзЗНАИжаЃЌЫЕУїHAЪЧШѕЕчНтжЪЕФЪЕбщЯжЯѓЪЧЃК
(ЬюађКХ)
A.МгШыСНжжЯЁЫсКѓЃЌСНИіЪдЙмЩЯЗНЕФЦјЧђЭЌЪБЙФЦ№ЃЌЧввЛбљДѓ
B.МгШыHAШмвККѓЃЌЪдЙмЩЯЗНЕФЦјЧђЙФЦ№Т§
C.МгШыЯЁбЮЫсКѓЃЌЪдЙмЩЯЗНЕФЦјЧђЙФЦ№Т§
(2) ввЭЌбЇЩшМЦЕФЪЕбщЕк ВНЃЌФмжЄУїИФБфЬѕМўШѕЕчНтжЪЦНКтЗЂЩњвЦЖЏЁЃМзЭЌбЇЮЊСЫНјвЛВНжЄУїШѕЕчНтжЪЕчРыЦНКтвЦЖЏЕФЧщПіЃЌЩшМЦШчЯТЪЕбщЃК
ЂйЪЙHAЕФЕчРыГЬЖШКЭc(H+)ЖММѕаЁЃЌc(A-)діДѓЃЌПЩдк0.1 molЁЄL-1ЕФHAШмвКжаЃЌбЁдёМгШы ЪдМС(бЁЬюЁАAЁБЁЂЁАBЁБЁЂЁАCЁБЛђЁАDЁБ,ЯТЭЌ)ЃЛ
ЂкЪЙHAЕФЕчРыГЬЖШМѕаЁЃЌc(H+)КЭc(A-)ЖМдіДѓЃЌПЩдк0.1 molЁЄL-1ЕФHAШмвКжаЃЌбЁдёМгШы ЪдМСЁЃ
A.NaAЙЬЬх(ПЩЭъШЋШмгкЫЎ)
B.1 molЁЄL-1 NaOHШмвК
C.1 molЁЄL-1 H2SO4
D.2 molЁЄL-1 HA
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃКЪЕбщЬт
ЪЕбщВтЖЈЫсМюжаКЭЕЮЖЈЧњЯпЪЧИпжаЛЏбЇЕФживЊЖЈСПЪЕбщЁЃЯТБэЪЧгУ0.10 mol/LЕФбЮЫсЕЮЖЈ 0.10 mol/L 20.00 mL NaOH ШмвКЪБЛёЕУЕФвЛаЉЯрЙиЪ§ОнЁЃЧыЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉЬюаДБэжаЂйЂкЖдгІЕФ pH ЃЈНсЙћБЃСєСНЮЛаЁЪ§ЃЉ
ЃЈ2ЃЉЯТЭМЪЧБОЪЕбщЕФЕЮЖЈЧњЯпЭМЁЃЧыИљОнИУЭМЃЌЫЕУїЧПЫсШмвКЕЮЖЈЧПМюШмвКЪБЃЌЮЊЪВУДМШПЩвдЪЙгУМзЛљГШзїЮЊжИЪОМСЃЌгжПЩвдЪЙгУЗгЬЊЪдвКзїжИЪОМСРДжИЪОЕЮЖЈжеЕуЃП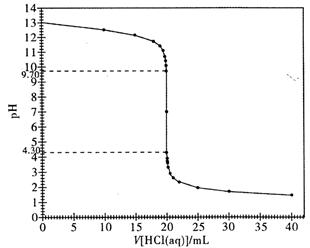
ЃЈ3ЃЉШєдкЕЮЖЈжеЕуЪБИЉЪгЖСЪ§ЃЌзюжеВтЕУЕФЧтбѕЛЏФЦШмвКЕФХЈЖШЛс ЃЈЬюЁАЦЋДѓЁБЁЂЁАЦЋаЁЁБЛђЁАУЛгагАЯьЁБЃЌЯТЭЌЃЉЃЛШєЕЮЖЈНсЪјЪБЃЌЕЮЖЈЙмМтзьаќгаАыЕЮБъзМбЮЫсЃЌзюжеВтЕУЕФЧтбѕЛЏФЦШмвКЕФХЈЖШЛсЃЛШєЪЂзАД§ВтвКЕФзЖаЮЦПЯДЕгИЩОЛКѓЃЌЮДИЩдяМДЪЂзАД§ВтвКЃЌзюжеВтЕУЕФЧтбѕЛЏФЦШмвКЕФХЈЖШЛс ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃКЪЕбщЬт
ФГбЇЩњгУвбжЊЮяжЪЕФСПХЈЖШЕФбЮЫсРДВтЖЈЮДжЊЮяжЪЕФСПХЈЖШЕФNaOHШмвКЪБЃЌбЁдёМзЛљГШзїжИЪОМСЁЃЧыЬюаДЯТСаПеАзЃК
ЃЈ1ЃЉгУБъзМЕФбЮЫсЕЮЖЈД§ВтЕФNaOHШмвКЪБЃЌзѓЪжЮеЫсЪНЕЮЖЈЙмЕФЛюШћЃЌгвЪжвЁЖЏзЖаЮЦПЃЌблОІзЂЪг ЃЌжБЕНзюКѓМгШывЛЕЮбЮЫсКѓЃЌШмвКгЩ ЩЋБфЮЊ ЃЌЧв ЮЊжЙЁЃ
ЃЈ2ЃЉЯТСаВйзїжаПЩФмЪЙЫљВтNaOHШмвКЕФХЈЖШЪ§жЕЦЋЕЭЕФЪЧ________
| AЃЎЫсЪНЕЮЖЈЙмЮДгУБъзМбЮЫсШѓЯДОЭжБНгзЂШыБъзМбЮЫс |
| BЃЎЕЮЖЈЧАЪЂЗХNaOHШмвКЕФзЖаЮЦПгУеєСѓЫЎЯДОЛКѓУЛгаИЩдя |
| CЃЎЫсЪНЕЮЖЈЙмдкЕЮЖЈЧАгаЦјХнЃЌЕЮЖЈКѓЦјХнЯћЪЇ |
| DЃЎЖСШЁбЮЫсЬхЛ§ЪБЃЌПЊЪМбіЪгЖСЪ§ЃЌЕЮЖЈНсЪјЪБИЉЪгЖСЪ§ |

| ЕЮЖЈ ДЮЪ§ | Д§ВтNaOHШмвКЕФЬхЛ§/mL | 0ЃЎ100 mol/LбЮЫсЕФЬхЛ§/mL | ||
| ЕЮЖЈЧАПЬЖШ | ЕЮЖЈКѓПЬЖШ | ШмвКЬхЛ§/mL | ||
| ЕквЛДЮ | 25ЃЎ00 | 0ЃЎ20 | 20ЃЎ22 | |
| ЕкЖўДЮ | 25ЃЎ00 | 0ЃЎ56 | 24ЃЎ54 | |
| ЕкШ§ДЮ | 25ЃЎ00 | 0ЃЎ42 | 20ЃЎ40 | |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃКЪЕбщЬт
ЫсМюжаКЭЕЮЖЈЪЧгІгУзюЖрЕФЕЮЖЈЁЃЯждквдЗгЬЊЮЊжИЪОМСЃЌгУвбжЊХЈЖШЕФNaOHШмвКШЅЕЮЖЈвЛЖЈЬхЛ§ЁЂЮДжЊХЈЖШЕФHClШмвКЁЃ
ЃЈ1ЃЉдкЕЮЖЈжаЃЌШєЪЕбщВйзїВЛЕБЛсЕМжТЪЕбщЮѓВюЁЃЯТСаВйзїЛсЪЙЪЕбщНсЙћЦЋИпЕФЪЧ ЁЃ
ЂйЫсЪНЕЮЖЈЙмЯДЕгКѓУЛгагУД§ВтвКШѓЯД
ЂкМюЪНЕЮЖЈЙмЯДЕгКѓУЛгагУБъзМвКШѓЯД
ЂлдкЕЮЖЈЙ§ГЬжаЃЌЯђзЖаЮЦПжаЬэМгеєСѓЫЎ
ЂмД§ВтвКДгзЖаЮЦПжаНІГі
ЂнЕЮЖЈЙ§ГЬжаЦ№ЪМЖСЪ§ЪБИЉЪгЃЌжеЕуКѓЖСЪ§ЪБбіЪг
ЃЈ2ЃЉдкЕЮЖЈЙмЪЙгУЪБЪзЯШгІИУНјааЕФВйзїЪЧ_______________________ЃЌЕЮЖЈЙ§ГЬжазѓЪжПижЦЕЮЖЈЙмЃЌгвЪжа§вЁзЖаЮЦПЃЌблОІзЂЪгзЖаЮЦПжаШмвКбеЩЋБфЛЏЃЌДяЕНЕЮЖЈжеЕуЕФЯжЯѓЪЧЃК______________________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃКЕЅбЁЬт
МюадЕчГиОпгаШнСПДѓЁЂЗХЕчЕчСїДѓЕФЬиЕуЃЌвђЖјЕУЕНЙуЗКгІгУЁЃаПЁЊУЬМюадЕчГивдЧтбѕЛЏМиШмвКЮЊЕчНтвКЃЌЕчГизмЗДгІЪНЮЊЃК Zn(s)+2MnO2(s)+H2O(l)==Zn(OH)2(s)+Mn2O3(s) ЃЌЯТСаЫЕЗЈДэЮѓЕФЪЧ( )
| AЃЎЕчГиЙЄзїЪБЃЌаПЪЇШЅЕчзг |
| BЃЎЕчГие§МЋЕФЕчМЋЗДгІЪНЮЊЃК2MnO2(s)+H2OЃЈ1ЃЉ+2eЁЊ=Mn2O3(s)+2OHЁЊ(aq) |
| CЃЎЕчГиЙЄзїЪБЃЌЕчзггЩе§МЋЭЈЙ§ЭтЕчТЗСїЯђИКМЋ |
| DЃЎЭтЕчТЗжаУПЭЈЙ§0.2molЕчзгЃЌаПЕФжЪСПРэТлЩЯМѕаЁ6.5g |
ВщПДД№АИКЭНтЮі>>
АйЖШжТаХ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com