
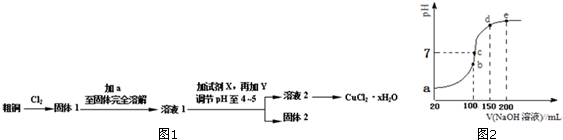
·ÖĪö ¢ń”¢ĘųĢåAæÉŹ¹³ĪĒåŹÆ»ŅĖ®±ä»ė×Ē£¬½įŗĻæ¹ĖįŅ©µÄÓŠŠ§³É·Ö£¬ÖŖøĆĘųĢåĪŖCO2£»
¢ņ”¢XÖŠŅ»¶Ø²»ŗ¬Na£¬ŅņĪŖNaµÄŃęÉ«ĪŖ»ĘÉ«£®
¢ó”¢øł¾ŻĢāøųŠÅĻ¢ÖŖµ÷½ŚpHÖĮ5”«6Ź±Éś³ÉµÄ°×É«³ĮµķĪŖAl£ØOH£©3£®
¢ō”¢¼ÓČė¹żĮæNaOHČÜŅŗ£¬³ĮµķBĶźČ«Čܽā£¬Ąė×Ó·½³ĢŹ½ĪŖ£ŗAl£ØOH£©3+OH-ØTAlO2-+2H2O£®
¢õ”¢¼ÓČėNaOHČÜŅŗµ÷½ŚpHÖĮ12£¬ÓŠ°×É«³Įµķ²śÉś£¬Ōņ³ĮµķCĪŖMg£ØOH£©2£®
¾Ż“Ė·ÖĪö£®
½ā“š ½ā£ŗ¢ń”¢ĘųĢåAæÉŹ¹³ĪĒåŹÆ»ŅĖ®±ä»ė×Ē£¬½įŗĻæ¹ĖįŅ©µÄÓŠŠ§³É·Ö£¬ÖŖøĆĘųĢåĪŖCO2£»
¢ņ”¢XÖŠŅ»¶Ø²»ŗ¬Na£¬ŅņĪŖNaµÄŃęÉ«ĪŖ»ĘÉ«£®
¢ó”¢øł¾ŻĢāøųŠÅĻ¢ÖŖµ÷½ŚpHÖĮ5”«6Ź±Éś³ÉµÄ°×É«³ĮµķĪŖAl£ØOH£©3£®
¢ō”¢¼ÓČė¹żĮæNaOHČÜŅŗ£¬³ĮµķBĶźČ«Čܽā£¬Ąė×Ó·½³ĢŹ½ĪŖ£ŗAl£ØOH£©3+OH-ØTAlO2-+2H2O£®
¢õ”¢¼ÓČėNaOHČÜŅŗµ÷½ŚpHÖĮ12£¬ÓŠ°×É«³Įµķ²śÉś£¬Ōņ³ĮµķCĪŖMg£ØOH£©2£»
£Ø1£©ĘųĢåAæÉŹ¹³ĪĒåŹÆ»ŅĖ®±ä»ė×Ē£¬½įŗĻæ¹ĖįŅ©µÄÓŠŠ§³É·Ö£¬ÖŖøĆĘųĢåĪŖCO2£¬
¹Ź“š°øĪŖ£ŗCO2£»
£Ø2£©Ņ»¶Ø²»ŗ¬Na£¬ŅņĪŖNaµÄŃęÉ«ĪŖ»ĘÉ«£¬¹Ź“š°øĪŖ£ŗNa£»
£Ø3£©µ÷½ŚpHÖĮ5”«6Ź±Éś³ÉµÄ°×É«³ĮµķĪŖAl£ØOH£©3£¬NH3•H20ĪŖČõµē½āÖŹ£¬Ąė×Ó·½³ĢŹ½ÖŠÓ¦Š“ĪŖ»ÆѧŹ½£¬¹Ź“š°øĪŖ£ŗAl3++3NH3•H20ØTAl£ØOH£©3”ż+3NH4+£»
£Ø4£©Al£ØOH£©3ĪŖĮ½ŠŌĒāŃõ»ÆĪļ£¬ÄÜČÜÓŚĒæ¼ī£¬¼ÓČė¹żĮæNaOHČÜŅŗ£¬Al£ØOH£©3³ĮµķĶźČ«Čܽā£¬Ąė×Ó·½³ĢŹ½ĪŖ£ŗAl£ØOH£©3+OH-ØTAlO2-+2H2O£¬
¹Ź“š°øĪŖ£ŗAl£ØOH£©3+OH-ØTAlO2-+2H2O£»
£Ø5£©¼ÓČėNaOHČÜŅŗµ÷½ŚpHÖĮ12£¬ÓŠ°×É«³Įµķ²śÉś£¬Ōņ³ĮµķCĪŖMg£ØOH£©2£¬
¹Ź“š°øĪŖ£ŗMg £ØOH£©2£®
µćĘĄ ±¾Ģāæ¼²éæ¹ĖįŅ©³É·ÖµÄĢ½¾æŹµŃ锢ĀĮµÄ»ÆŗĻĪļµÄŠŌÖŹ£¬²ąÖŲÓŚæ¼²éѧɜµÄ·ÖĪöÄÜĮ¦ŗĶ¶Ō»ł“”ÖŖŹ¶µÄÓ¦ÓĆÄÜĮ¦”¢¼°ŹµŃéĢ½¾æÄÜĮ¦£¬ĢāÄæÄѶČÖŠµČ£®


 ½šŌæ³×ŹŌ¾ķĻµĮŠ“š°ø
½šŌæ³×ŹŌ¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ÓĆHNO3³żČ„Ķ·ŪÖŠ»ģŌÓµÄZn | |
| B£® | ĶØČėCl2£¬³żČ„Fe2£ØSO4£©3ČÜŅŗÖŠµÄFeSO4 | |
| C£® | Ķعż±„ŗĶŹ³ŃĪĖ®£¬³żČ„Cl2ÖŠµÄHCl | |
| D£® | Ķعż±„ŗĶNa2CO3ČÜŅŗ£¬³żČ„CO2ÖŠµÄSO2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¹āµ¼ĻĖĪ¬ŹĒŠÅĻ¢Éē»į±Ų²»æÉÉŁµÄÓŠ»śŗĻ³É²ÄĮĻ | |
| B£® | Ź³ŃĪ¼ÓµāŹµÖŹŹĒŌŚŹ³ŃĪÖŠ¼ÓČėKIO3 | |
| C£® | ŗ½Ģģ·É»śÉĻµÄĢÕ“É·Ą»¤Ę¬ŹōÓŚŠĀŠĶĪŽ»ś·Ē½šŹō²ÄĮĻ | |
| D£® | ·ŁÉÕĄ¬»ų»į²śÉś“óĮæĪŪČ¾æÕĘųµÄĪļÖŹ£¬¹Ź²»ŅĖ²ÉÓĆ“Ė·Ø |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Na2SO4ÓėNaAlO2×é³ÉµÄ»ģŗĻĪļ | B£® | KAl£ØSO4£©2 | ||
| C£® | Al2£ØSO4£©3 | D£® | NH4Al£ØSO4£©2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 ŌŚŅ»¶ØĢõ¼žĻĀ£¬N2ŗĶH2ĶźČ«·“Ӧɜ³É1molNH3·ÅČČ46.0kJČČĮ森Š“³ö°±·Ö½āĪŖĒāĘųŗĶµŖĘųµÄČČ»Æѧ·½³ĢŹ½2NH3£Øg£©=N2£Øg£©+3H2£Øg£©”÷H=+92KJ/mol
ŌŚŅ»¶ØĢõ¼žĻĀ£¬N2ŗĶH2ĶźČ«·“Ӧɜ³É1molNH3·ÅČČ46.0kJČČĮ森Š“³ö°±·Ö½āĪŖĒāĘųŗĶµŖĘųµÄČČ»Æѧ·½³ĢŹ½2NH3£Øg£©=N2£Øg£©+3H2£Øg£©”÷H=+92KJ/mol²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | H2SO4µĪČėNaAlO2ČÜŅŗÖŠ | B£® | Ba£ØOH£©2ČÜŅŗµĪČėAl2£ØSO4£©3ČÜŅŗÖŠ | ||
| C£® | Al2£ØSO4£©3ČÜŅŗµĪČėNaOHČÜŅŗÖŠ | D£® | °±Ė®µĪČėAl2£ØSO4£©3ČÜŅŗÖŠ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŗģ×ŲÉ«µÄNO2ĘųĢå¼ÓŃ¹ŗóŃÕÉ«ĻȱäÉīŌŁ±äĒ³ | |
| B£® | “ņæŖĘūĖ®Ę棬擵½ÓŠ“óĮæµÄĘųÅŻŅŻ³ö | |
| C£® | ¶žŃõ»ÆĮņ×Ŗ»ÆĪŖČżŃõ»ÆĮņŹ±Ōö¼ÓæÕĘųµÄĮæŅŌĢįø߶žŃõ»ÆĮņµÄ×Ŗ»ÆĀŹ | |
| D£® | H2”¢I2”¢HI»ģŗĻĘųĢå¼ÓŃ¹ŗóŃÕÉ«±äÉī |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com