
ÓŠĻĀĮŠ¾ÅÖÖ¾§Ģå£ŗA.Ė®¾§””B£®±ł“×Ėį””C£®Ńõ»ÆĆ¾””D£®°×Į×””E£®¾§Ģåė²””F£®ĀČ»Æļ§””G£®¹żŃõ»Æ¼Ų””H£®½šøÕŹÆ””I£®Ć¾
(1)ŹōÓŚŌ×Ó¾§ĢåµÄ»ÆŗĻĪļŹĒ________£¬Ö±½ÓÓÉŌ×Ó¹¹³ÉµÄ¾§ĢåŹĒ________£¬Ö±½ÓÓÉŌ×Ó
¹¹³ÉµÄ·Ö×Ó¾§ĢåŹĒ________”£
(2)Óɼ«ŠŌ·Ö×Ó¹¹³ÉµÄ¾§ĢåŹĒ________£¬ŗ¬ÓŠ¹²¼Ū¼üµÄĄė×Ó¾§ĢåŹĒ________£¬ŹōÓŚ·Ö×Ó¾§
ĢåµÄµ„ÖŹŹĒ________”£
(3)ŹÜČČČŪ»Æŗó»Æѧ¼ü²»·¢Éś±ä»ÆµÄŹĒ__________£¬ŠčæĖ·ž¹²¼Ū¼üµÄŹĒ________£¬ŠčŅŖæĖ·ž½šŹō¼üµÄŹĒ________”£

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĒāĘųŹĒÖŲŅŖ¶ų½ą¾»µÄÄÜŌ“”£ŅŖĄūÓĆĒāĘų×÷ĪŖÄÜŌ“£¬±ŲŠė½ā¾öŗĆ°²Č«ÓŠŠ§µŲ“¢“ęĒāĘųµÄĪŹĢā”£»Æѧ¼ŅŃŠ¾æ³öĄūÓĆŗĻ½š“¢“ęĒāĘųµÄ·½·Ø£¬ĘäÖŠļē(La)Äų(Ni)ŗĻ½šŹĒŅ»ÖÖ“¢Ēā²ÄĮĻ£¬ÕāÖÖŗĻ½šµÄ¾§Ģå½į¹¹ŅŃ¾²ā¶Ø£¬Ę仳±¾½į¹¹µ„ŌŖČēĶ¼ĖłŹ¾£¬ŌņøĆŗĻ½šµÄ»ÆѧŹ½æɱķŹ¾ĪŖ(””””)
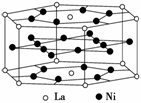
A£®LaNi5 B£®LaNi
C£®La14Ni24 D£®La7Ni12
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ÓĆ¼Ūµē×Ó¶Ō»„³āĄķĀŪŌ¤²āH2SŗĶBF3µÄĮ¢Ģå½į¹¹£¬Į½øö½įĀŪ¶¼ÕżČ·µÄŹĒ(””””)
A£®Ö±ĻߊĪ£»Čż½Ē׶ŠĪ B£®VŠĪ£»Čż½Ē׶ŠĪ
C£®Ö±ĻߊĪ£»Ę½ĆęČż½ĒŠĪ D£®VŠĪ£»Ę½ĆęČż½ĒŠĪ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŌŚµ„ÖŹµÄ¾§ĢåÖŠŅ»¶Ø²»“ęŌŚµÄĪ¢Į£ŹĒ(””””)
A£®Ō×Ó B£®·Ö×Ó
C£®ŅõĄė×Ó D£®ŃōĄė×Ó
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
½šŹō¾§Ģ唢Ąė×Ó¾§ĢåŗĶ·Ö×Ó¾§Ģå²ÉČ”Ćܶѻż·½Ź½µÄŌŅņŹĒ(””””)
A£®¹¹³É¾§ĢåµÄĪ¢Į£¾łæÉŹÓĪŖŌ²Ēņ
B£®½šŹō¼ü”¢Ąė×Ó¼ü”¢·Ö×Ó¼ä×÷ÓĆĮ¦¾łĪŽ±„ŗĶŠŌŗĶ·½ĻņŠŌ
C£®ČżÖÖ¾§ĢåµÄ¹¹³ÉĪ¢Į£ĻąĶ¬
D£®ČżÖÖ¾§ĢåµÄ¹¹³ÉĪ¢Į£¶ąÉŁ¼°Ļą»„×÷ÓĆĮ¦ĻąĶ¬
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
0.5 mol AŌŖĖŲµÄ×īøß¼ŪĄė×Ó±»»¹Ō³ÉÖŠŠŌŌ×ÓŹ±£¬µĆµ½6.02”Į1023øöµē×Ó£¬ĖüµÄµ„ÖŹĶ¬ŃĪĖį³ä·Ö·“Ó¦Ź±£¬·Å³ö0.02 g ĒāĘų£¬ÓĆČ„0.4 gµ„ÖŹA”£BŌŖĖŲµÄŌ×ÓŗĖĶāµē×Ó²ćŹżÓėAĻąĶ¬£¬ĒŅBŌŖĖŲŠĪ³ÉµÄµ„ÖŹŹĒÉīŗģ×ŲÉ«ŅŗĢ唣
(1)Š“³öĮ½ÖÖŌŖĖŲµÄĆū³Ę£ŗA________£¬B________£»
(2)ÓĆ½į¹¹Ź¾ŅāĶ¼±ķŹ¾Į½ÖÖŌŖĖŲµÄ³£¼ūĄė×Ó___________”£
(3)Öø³öAÓėBŠĪ³É»ÆŗĻĪļµÄ»Æѧ¼üµÄĄąŠĶ£ŗ
________________________________________________________________________ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
¾ßÓŠĻĀĮŠµē×ÓÅŲ¼µÄŌ×ÓÖŠ×īÄŃŠĪ³ÉĄė×Ó¼üµÄŹĒ(””””)
A£®1s22s22p2 B£®1s22s22p5
C£®1s22s22p63s2 D£®1s22s22p63s1
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĢģȻӊ»śøß·Ö×Ó»ÆŗĻĪļAŹĒĀĢÉ«Ö²Īļ¹āŗĻ×÷ÓĆµÄ²śĪļ£¬Ö÷ŅŖ“ęŌŚÓŚÖ²ĪļµÄÖÖ×ÓŗĶæéøłĄļ£¬ÄÜ·¢ÉśČēĻĀ±ä»Æ(A”«F¾ł·Ö±š“ś±ķŅ»ÖÖĪļÖŹ£¬æņĶ¼ÖŠµÄ²æ·Ö²śĪļŅŃĀŌČ„£¬ÓÉBÉś³ÉCµÄĶ¬Ź±»¹ÓŠCO2Éś³É)”£
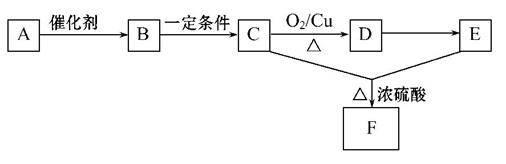 £Ø1£©ÓÉAÉś³ÉBµÄ»Æѧ·½³ĢŹ½ĪŖ__________________”£
£Ø1£©ÓÉAÉś³ÉBµÄ»Æѧ·½³ĢŹ½ĪŖ__________________”£
£Ø2£©B”¢D¾łŗ¬ÓŠµÄ¹ŁÄÜĶÅŹĒ________£¬Éč¼ĘŹµŃ飬ÓĆČõŃõ»Æ¼Į¼ģŃéB·Ö×Ó½į¹¹ÖŠ“ęŌŚøĆ¹ŁÄÜĶÅ(ŅŖĒó£ŗ¼ņŅŖĆčŹö¼ģŃé¹ż³Ģ£»ŅŌĻĀ²½ÖčæÉŅŌ²»ĢīĀś)”£
¢Ł_____________________________________________________________£»
¢Ś_____________________________________________________________£»
¢Ū____________________________________________________________”£
£Ø3£©“ÓAµ½F·¢ÉśµÄ·“Ó¦ĄąŠĶÓŠ________”£
A.Ńõ»Æ””””””B.õ„»Æ””””””C.¼Ó³É””””””D.Ė®½ā
£Ø4£©Š“³öCµ½DĖł·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½_________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
NA±ķŹ¾°¢·ü¼ÓµĀĀŽ³£ŹżµÄŹżÖµ£¬ĻĀĮŠĖµ·Ø²»ÕżČ·µÄŹĒ(””””)
A£®½«1 mol NH4NO3ČÜÓŚŅ»¶ØÅØ¶ČµÄĻ”°±Ė®ÖŠ£¬ČÜŅŗ³ŹÖŠŠŌ£¬Čō²»æ¼ĀĒ»Ó·¢£¬ČÜŅŗÖŠŅ»¶Øŗ¬ÓŠNAøöNH
B£®1 mol·L£1 CH3COOHÓė1 mol·L£1 CH3COONaČÜŅŗµČĢå»ż»ģŗĻ£¬ČÜŅŗÖŠCH3COOHŗĶCH3COO£µÄ×ÜŹżĪŖ2NA
C£®³£ĪĀ³£Ń¹ĻĀ£¬3.6 g H2OÖŠŗ¬ÓŠµē×ÓŹżĪŖ2NA
D£®ŗ¬ÓŠ2NAøöŃõŌ×ÓµÄŃõĘųÓė³ōŃõµÄ»ģŗĻĘųĢåµÄÖŹĮæĪŖ32 g
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com