
����ʵ��������ʵ����ʵ��������ȷ���м������� ��
���ð�ˮ��ϴ����������Ӧ���Թܣ�
������һ�����ʵ���Ũ�ȵ���Һʱ������ʱ��������ҺŨ��ƫ�͡�
����ʪ���pH��ֽ�ⶨϡ�����pH��
���ü�ʽ�ζ�����ȡ20.00 mL 0.1mol/LKMnO4��Һ
�ݽ�Na2CO3�����ڴ������м������ڣ�
������������Һմ��Ƥ���ϣ�������NaOHϡ��Һ��ϴ
������ڵ�NaOH��Һ�еμ�FeCl3��Һ���Ʊ�Fe(OH)3����
������FeSO4��Һʱ��������������ۺ�ϡ����
A��5 B��3 C�� 2 D��1
C
�������������ʲ������ڰ�ˮ�У��ٲ���ȷ������ʱ����������ƿ����Һ���ƫ��Ũ��ƫС������ȷ��pH��ֽ������ҺpHֵʱ������������ʪ���۲���ȷ�����������ҺӦ������ʽ�ζ�����ȡ���ܲ���ȷ���������к��ж������裬�������ܺ�̼���Ʒ�Ӧ���ݲ���ȷ������ȷ��Ӧ���þƾ�ϴ�ӣ�ѡ��߲���ȷ��Ӧ���Ƿ��ڵ�����ˮ�У�ѡ�����ȷ�����Ƿ�ֹ��������ϡ�����ֹˮ�⣬���Դ�ѡC��


 ���ٴ�����ɽ����ϵ�д�
���ٴ�����ɽ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ʵ��������ʵ����ʵ��������ȷ���� ������ţ���
�����Թ��еμ�Һ��ʱ��Ϊ��ʹҺ��ε��Թ���Ӧ����ͷ�ι������Թ��У�
��һС������Ƽ���ˮ�к�Ѹ���۳�С��ͣ����ˮ���ζ���������֨֨����������
������100mL1.00mol/L��NaCl��Һʱ������������ƽ��ȡ5.85g NaCl���壻
������ܺ���SO42����SO32������Һ�м�����������ᣬ�ټ���Ba(NO3)2��Һ���ɼ���SO42���Ĵ��ڣ�
������NaCl��Һ�Եõ�NaCl����ʱ�����ؽ���Һ���ɣ�
����100��ʱ��NaOHϡ��Һ�еμӱ��͵�FeCl3��Һ�����Ʊ�Fe(OH)3���壻
����ͼ���ɹ۲쵽���������Ƶ�ָ��ƫת��
����AlCl3��Һ�еμ�NaOH��Һ����NaOH��Һ�еμ�AlCl3�� Һ��������ͬ��
������0.1mol��L��1�Ĵ�����Һ����pH��ֽ�ⶨ����Һ��pH����
��ȷ�IJ�����
����Ϊ����ҺpH�ķ�Χһ������ ֮�䡣�������һ����ʵ�鷽��֤��������Һ�ʼ�������CO32������ģ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�갲����ѧ�߶�������ϰ��ѧ�����ģ� ���ͣ�ʵ����
������ʵ��������ʵ����ʵ��������ȷ���� ������ţ���
�����Թ��еμ�Һ��ʱ��Ϊ��ʹҺ��ε��Թ���Ӧ����ͷ�ι������Թ��У�
��һС������Ƽ���ˮ�к�Ѹ���۳�С��ͣ����ˮ���ζ���������֨֨����������
������100mL1.00mol/L��NaCl��Һʱ������������ƽ��ȡ5.85g NaCl���壻
������ܺ���SO42����SO32������Һ�м�����������ᣬ�ټ���Ba(NO3)2��Һ���ɼ���SO42���Ĵ��ڣ�
������NaCl��Һ�Եõ�NaCl����ʱ�����ؽ���Һ���ɣ�
����100��ʱ��NaOHϡ��Һ�еμӱ��͵�FeCl3��Һ�����Ʊ�Fe(OH)3���壻
����ͼ���ɹ۲쵽���������Ƶ�ָ��ƫת��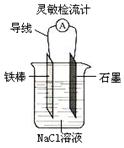
����AlCl3��Һ�еμ�NaOH��Һ����NaOH��Һ�еμ�AlCl3�� Һ��������ͬ��
������0.1mol��L��1�Ĵ�����Һ����pH��ֽ�ⶨ����Һ��pH����
��ȷ�IJ�����
����Ϊ����ҺpH�ķ�Χһ������ ֮�䡣�������һ����ʵ�鷽��֤��������Һ�ʼ�������CO32������ģ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�갲����ѧ�߶�������ϰ��ѧ�����ģ� ���ͣ�ʵ����
������ʵ��������ʵ����ʵ��������ȷ���� ������ţ���
�����Թ��еμ�Һ��ʱ��Ϊ��ʹҺ��ε��Թ���Ӧ����ͷ�ι������Թ��У�
��һС������Ƽ���ˮ�к�Ѹ���۳�С��ͣ����ˮ���ζ���������֨֨����������
������100mL1.00mol/L��NaCl��Һʱ������������ƽ��ȡ5.85g NaCl���壻
������ܺ���SO42����SO32������Һ�м�����������ᣬ�ټ���Ba(NO3)2��Һ���ɼ���SO42���Ĵ��ڣ�
������NaCl��Һ�Եõ�NaCl����ʱ�����ؽ���Һ���ɣ�
����100��ʱ��NaOHϡ��Һ�еμӱ��͵�FeCl3��Һ�����Ʊ�Fe(OH)3���壻
����ͼ���ɹ۲쵽���������Ƶ�ָ��ƫת��
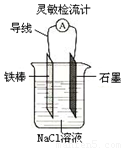
����AlCl3��Һ�еμ�NaOH��Һ����NaOH��Һ�еμ�AlCl3�� Һ��������ͬ��
������0.1mol��L��1�Ĵ�����Һ����pH��ֽ�ⶨ����Һ��pH����
��ȷ�IJ�����
����Ϊ����ҺpH�ķ�Χһ������ ֮�䡣�������һ����ʵ�鷽��֤��������Һ�ʼ�������CO32������ģ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����Թ��еμ�Һ��ʱ��Ϊ��ʹҺ��ε��Թ���Ӧ����ͷ�ι������Թ��У�
������100 mL 1.00mol/L��NaCl��Һʱ������������ƽ��ȡ5.85 gNaCl���壻
����100��ʱ��NaOHϡ��Һ�еμӱ��͵�FeCl3��Һ�����Ʊ�Fe(OH)3���壻
�������Ȼ�����Һʱ���Ƚ��Ȼ�����������������ܽ⣬Ȼ���ˮϡ�ͣ�
�ݷ�Һ©����Һʱ���Ƚ��²��Һ����¿�������Ȼ���ٴ��¿������ϲ��Һ��
�ⶨij��ҺpHʱ��ȡһС��������ֽ���벣��Ƭ�ϣ��ò�����պȡ��Һ��
����ֽ���в�������pH��ֽ��ɫ���Ա�(2)����ͼ��ʾ��ʵ��װ���У�ʢ������ˮ��ˮ����������ձ���С�ձ����������ͭƬ����Ũ���ᣬС�ձ����浹��һ���ձ�����ش��������⣺
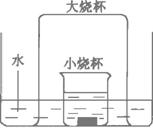
��.ʵ������У��۲쵽����Ҫ�����ǣ�
��ͭƬ����������ݣ�ͭƬ��С������ʧ��
��С�ձ�����Һ����ɫ�����ɫ��
��________________________________________________��
��________________________________________________��
��.�ø�װ����ͭ��Ũ���ᷴӦ��ʵ�飬��ͻ�����ŵ���______________��
��.��Ҫ��֤���յõ����������������ķ�����______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ʵ��������ʵ����ʵ��������ȷ���� ������ţ���
�����Թ��еμ�Һ��ʱ��Ϊ��ʹҺ��ε��Թ���Ӧ����ͷ�ι������Թ��У�
��һС������Ƽ���ˮ�к�Ѹ���۳�С��ͣ����ˮ���ζ���������֨֨����������
������100mL1.00mol/L��NaCl��Һʱ������������ƽ��ȡ5.85g NaCl���壻
������ܺ���SO42����SO32������Һ�м�����������ᣬ�ټ���Ba(NO3)2��Һ���ɼ���SO42���Ĵ��ڣ�
������NaCl��Һ�Եõ�NaCl����ʱ�����ؽ���Һ���ɣ�
����100��ʱ��NaOHϡ��Һ�еμӱ��͵�FeCl3��Һ�����Ʊ�Fe(OH)3���壻
����ͼ���ɹ۲쵽���������Ƶ�ָ��ƫת��
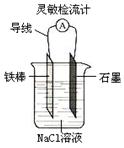
����AlCl3��Һ�еμ�NaOH��Һ����NaOH��Һ�еμ�AlCl3�� Һ��������ͬ��
������0.1mol��L��1�Ĵ�����Һ����pH��ֽ�ⶨ����Һ��pH����
��ȷ�IJ�����
����Ϊ����ҺpH�ķ�Χһ������ ֮�䡣�������һ����ʵ�鷽��֤��������Һ�ʼ�������CO32������ģ� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com