
| ķĄĀėÖŹĮæ/g | 50 | 20 | 20 | 10 | 5 |
| ³ĘĮæ£ØČ”ÓĆķĄĀė¹ż³Ģ£© |

·ÖĪö £Ø1£©øł¾ŻÅäÖĘŅ»¶ØĪļÖŹµÄĮæÅØ¶ČµÄČÜŅŗ²½ÖčŃ”ÓĆŅĒĘ÷£¬Č»ŗóÅŠ¶Ļ»¹Č±ÉŁµÄŅĒĘ÷£»
£Ø2£©ŅĄ¾Żm=CVM¼ĘĖćČÜÖŹµÄÖŹĮ棻
£Ø3£©¢ŁŅĄ¾ŻĶŠÅĢĢģĘ½Ź¹ÓĆ·½·Ø£ŗ×óĪļÓŅĀė½ā“š£»
¢ŚĶŠÅĢĢģĘ½µÄ¾«Č·¶ČĪŖ0.1g£¬·Ö¶ČÅĢµÄÖøÕėĘ«ÓŅ½įŗĻĢģĘ½µÄ³ĘĮæŌĄķ¼°ÕżČ·Ź¹ÓĆ·½·Ø½ā“š£»
£Ø4£©ČܽāĪļÖŹŹ¹ÓĆ²£Į§°ōæÉŅŌ¼ÓæģČܽāĖŁĀŹ£»
£Ø5£©ŅĘŅŗŹ±ĪŖ·ĄÖ¹ŅŗĢåĮ÷³ö£¬Ó¦ÓĆ²£Į§°ōŅżĮ÷£»Ļ“µÓÉÕ±2“Ī”«3“ĪŹĒĪŖĮĖ±£Ö¤ČÜÖŹČ«²æ×ŖŅĘÖĮČŻĮæĘæÖŠ£»
£Ø6£©ŅĄ¾Ż¶ØČŻµÄÕżČ·²Ł×÷½ā“š£»
£Ø7£©·ÖĪö²»µ±²Ł×÷¶ŌČÜÖŹµÄĪļÖŹµÄĮæŗĶČÜŅŗĢå»żµÄÓ°Ļģ£¬ŅĄ¾ŻC=$\frac{n}{V}$½ųŠŠĪó²ī·ÖĪö£®
½ā“š ½ā£ŗ£Ø1£©ÅäÖĘ480mL 0.5mol•L-1µÄNaOHČÜŅŗ£¬Ó¦Ń”Ōń500mlČŻĮæĘ棬Źµ¼ŹÉĻÅäÖʵďĒ500mL 0.5mol/LµÄĒāŃõ»ÆÄĘČÜŅŗ£¬ÅäÖĘ²½ÖčĪŖ£ŗ¼ĘĖć”¢³ĘĮ攢Čܽā”¢ĄäČ“”¢×ŖŅĘ”¢Ļ“µÓ”¢¶ØČŻ”¢Ņ”ŌČµČ£¬ŠčŅŖŹ¹ÓƵÄŅĒĘ÷ÓŠ£ŗĶŠÅĢĢģĘ½”¢²£Į§°ō”¢ÉÕ±”¢Ņ©³×”¢500mLČŻĮæĘ攢½ŗĶ·µĪ¹ÜµČ£¬»¹Č±ÉŁµÄ²£Į§ŅĒĘ÷ĪŖ£ŗ500mLČŻĮæĘ攢½ŗĶ·µĪ¹Ü£¬
¹Ź“š°øĪŖ£ŗ500mLČŻĮæĘ棻½ŗĶ·µĪ¹Ü£»
£Ø2£©ÅäÖĘ480mL 0.5mol•L-1µÄNaOHČÜŅŗ£¬ŠčŅŖČÜÖŹĒāŃõ»ÆÄĘÖŹĮæ=0.5L”Į0.5mol•L-1”Į40g/mol=10.0g£»
¹Ź“š°øĪŖ£ŗ10.0g£»
£Ø3£©¢Ł³ĘĮæ¹ż³ĢÖŠNaOH¹ĢĢåÓ¦·ÅÓŚŠ”ÉÕ±ÖŠ²¢·ÅŌŚĢģĘ½µÄ×óÅĢ£»
¹Ź“š°øĪŖ£ŗ×óÅĢ£»
¢Ś·Ö¶ČÅĢµÄÖøÕėĘ«ÓŅ£¬ĖµĆ÷ÓŅ±ßÖŲ£¬×óÅĢøßÓŚÓŅÅĢ£¬×óÅĢÖŹĮæŠ”ÓŚÓŅÅĢ£»ĶŠÅĢĢģĘ½µÄ¾«Č·¶ČĪŖ0.1g£¬ĖłŅŌ
Š”ÉÕ±µÄÖŹĮæ32.6 g£»
ÓĆĶŠÅĢĢģĘ½×¼Č·³ĘĘäÖŹĮ棬ĻČŌŚÓŅÅĢ¼ÓČė50gķĄĀė£¬ÖŹĮæ¹ż“󣬱ķŹ¾ĪŖ£ŗ50g”ż”ü£»Č»ŗó»»20gķĄĀė£¬ķĄĀėÖŹĮæ²»×ć£¬±ķŹ¾ĪŖ£ŗ20g”ż£»ŌŁ¼Ó20gķĄĀė£¬20gķĄĀėÖŹĮæ¹ż“󣬱ķŹ¾ĪŖ£ŗ”ż”ü£»»»10gķĄĀė£¬10gķĄĀėÖŹĮæ²»×ć£¬±ķŹ¾ĪŖ£ŗ10g”ż£»ŌŁ¼Ó5gķĄĀė£¬5gķĄĀėÖŹĮæ¹ż“ó£¬ÄĆĻĀ5gķĄĀė£¬±ķŹ¾ĪŖ£ŗ5g”ż”ü£»×īŗóŅʶÆÓĪĀėµ½2.6g½ųŠŠµ÷Ę½ĢģĘ½£¬±ź³ßÉĻ»³öÓĪĀėµÄĪ»ÖĆĪŖ£ŗ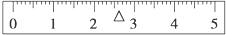 £¬
£¬
¹Ź“š°øĪŖ£ŗ×óÅĢ””Š”ÓŚ””32.6 g£»
| ķĄĀėÖŹĮæ/g | 50 | 20 | 20 | 10 | 5 |
| ³ĘĮæ£ØČ”ÓĆķĄĀė¹ż³Ģ£© | ”ż”ü | ”ż | ”ż”ü | ”ż | ”ż”ü |
 £»
£»µćĘĄ ±¾Ģāæ¼²éĮĖÅäÖĘŅ»¶ØĪļÖŹµÄĮæÅضČČÜŅŗ£¬Ć÷Č·ÅäÖĘŌĄķŗĶ¹ż³ĢŹĒ½āĢā¹Ų¼ü£¬×¢ŅāČŻĮæĘ攢ĶŠÅĢĢģĘ½µÄŹ¹ÓĆ·½·Ø£¬ĢāÄæÄŃ¶Č²»“ó£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¢Ł¢ß | B£® | ¢Ś¢Ü | C£® | ¢Ū¢Ż¢Ž | D£® | ¢Ś¢Ü¢Ż¢Ž |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
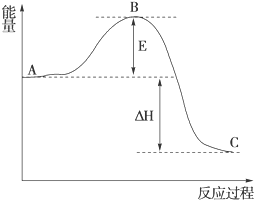 £Ø1£©ŌŚ25”ę”¢101kPaĻĀ£¬1g¼×ĶéĶźČ«Č¼ÉÕÉś³ÉCO2ŗĶŅŗĢ¬H2O£¬·Å³ö55kJµÄČČĮ棬Š“³ö±ķŹ¾¼×ĶéČ¼ÉÕČȵÄČČ»Æѧ·½³ĢŹ½£ŗCH4£Øg£©+2O2£Øg£©ØTCO2£Øg£©+H2O£Øl£©”÷H=-880kJ/mol£®
£Ø1£©ŌŚ25”ę”¢101kPaĻĀ£¬1g¼×ĶéĶźČ«Č¼ÉÕÉś³ÉCO2ŗĶŅŗĢ¬H2O£¬·Å³ö55kJµÄČČĮ棬Š“³ö±ķŹ¾¼×ĶéČ¼ÉÕČȵÄČČ»Æѧ·½³ĢŹ½£ŗCH4£Øg£©+2O2£Øg£©ØTCO2£Øg£©+H2O£Øl£©”÷H=-880kJ/mol£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 µÄ·Šµć±Č
µÄ·Šµć±Č øߣ¬ŌŅņ
øߣ¬ŌŅņ ŠĪ³É·Ö×ÓÄŚĒā¼ü£¬¶ų
ŠĪ³É·Ö×ÓÄŚĒā¼ü£¬¶ų ŠĪ³É·Ö×Ó¼äĒā¼ü£¬·Ö×Ó¼äĒā¼üŹ¹·Ö×Ó¼ä×÷ÓĆĮ¦Ōö“ó£®
ŠĪ³É·Ö×Ó¼äĒā¼ü£¬·Ö×Ó¼äĒā¼üŹ¹·Ö×Ó¼ä×÷ÓĆĮ¦Ōö“ó£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¢Ś¢Ū¢Ü¢Ż¢ą | B£® | ¢Ł¢Ū¢Ü¢Ż¢ą | C£® | ¢Ł¢Ū¢Ü¢ą | D£® | ŅŌÉĻ¾ł²»¶Ō |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 ŹµŃ銔×éĶ¬Ń§æ“µ½Ņ»Ōņ±ØµĄ£ŗijŌģÖ½³§Ī󽫲Ū³µÖŠĘÆ°×Ņŗ£ØNaClŗĶNaClOµÄ»ģŗĻŅŗ£©µ¹ČėŹ¢·Å±„ŗĶKAl£ØSO4£©2ČÜŅŗµÄ³ŲÖŠ£¬Ōģ³ÉÖŠ¶¾ŹĀ¼ž£¬øĆŠ”×éĶ¬Ń§ĪŖĢ½¾æÖŠ¶¾ŌŅņ½ųŠŠĮĖČēĻĀŹµŃé£ŗ
ŹµŃ銔×éĶ¬Ń§æ“µ½Ņ»Ōņ±ØµĄ£ŗijŌģÖ½³§Ī󽫲Ū³µÖŠĘÆ°×Ņŗ£ØNaClŗĶNaClOµÄ»ģŗĻŅŗ£©µ¹ČėŹ¢·Å±„ŗĶKAl£ØSO4£©2ČÜŅŗµÄ³ŲÖŠ£¬Ōģ³ÉÖŠ¶¾ŹĀ¼ž£¬øĆŠ”×éĶ¬Ń§ĪŖĢ½¾æÖŠ¶¾ŌŅņ½ųŠŠĮĖČēĻĀŹµŃé£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com