
���� ��ʵ��Ŀ���Dzⶨ����������������ȡ�ķ�����ʹ��Ʒ�ܽ⡢��Ӧ������������������Ȼ��ͨ������������������������������
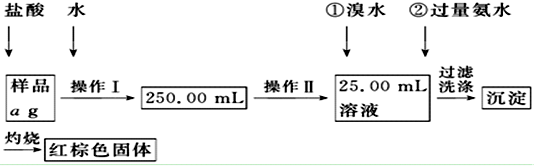
��1����ͼ��֪������I�ǽ��������ᷴӦ����Һϡ�ͳ�250.00mL��Һ������Ҫ250mL������ƿ������II��ȷ��ȡ25.00mL��ϡ�ͺ����Һ����Ӧ��Ҫ�ζ��ܣ�
��2������ˮĿ������+2������Ϊ+3�ۣ��Ӱ�ˮ����ʹ+3�������ת��ΪFe��OH��3������
��3����Һ�д����廯泥����������ữ����������Һ�������һ��ϴ��Һ���Ƿ���������ӣ�
��4�����ȷֽ����õ�������Fe2O3��������Ϊ��W2-W1��g������ȥ20mL��Һ����100mL��Һ���Եõ�Fe2O3����Ϊ5��W2-W1��g�����ݻ�ѧʽ������Ԫ�ص����������������������Ķ������ԭ��������Ʒ����Ԫ�ص�����������
��� �⣺��1��������һ�����ʵ���Ũ�ȵ���Һ����Ҫ�������У���Ͳ����ͷ�ιܡ��ձ�����������һ����������ƿ������I�ǽ��������ᷴӦ����Һϡ�ͳ�250.00mL��Һ������Ҫ250mL������ƿ�������IIΪ�ζ������������õζ��ܣ�ѡD��
�ʴ𰸣�250mL����ƿ����ͷ�ιܣ�D��
��2����Br2���������ԣ�������Fe2+��2Fe2++Br2=2Fe3++2Br-��Ϊ��ʹFe3+��ֳ�������ˮҪ�������γ��������������������յõ����������壬
�ʴ�Ϊ��2Fe2++Br2=2Fe3++2Br-��Ϊ��ʹFe3+��ֳ�����
��3����Һ�д����廯泥����������ữ����������Һ�������һ��ϴ��Һ���Ƿ���������ӣ����жϳ����Ƿ�ϴ����
�ʴ�Ϊ��ȡ���һ��ϴ��Һ�����Թ��У��μ�������������Һ������ɫ�������ɣ���֤��ϴ�Ӹɾ���
��4������Ԫ�������غ㣬������ɫ�����е���������Ʒ������Fe2O3������ΪW2-W1g�����ڲμӷ�Ӧ����Һֻȡ������Һ��$\frac{1}{10}$�������Ԫ�ص�����Ϊ10����W2-W1��g��$\frac{112}{160}$����Ʒ����Ԫ�ص�����������$\frac{10��112��{W}_{2}-{W}_{��}��}{160a}$��100%=$\frac{1120��{W}_{2}-{W}_{1}��}{160a}$��100%������������Ϊ��ȫ�ֽ⣬�������Ԫ�ص�����ƫ�ߣ����ղ����Ľ��ƫ��
�ʴ�Ϊ��$\frac{1120��{W}_{2}-{W}_{1}��}{160a}$��100%������ʱ����δ��ַ�Ӧ��ΪFe2O3��
���� ���⿼����Һ���ơ����Ӽ��顢��ʵ�������������ʵ�鷽�������ۡ���ѧ����ȣ��Ѷ��еȣ�����ⶨԭ���ǽ���Ĺؼ����Ƕ���ѧ֪ʶ���ۺ����ã���Ҫѧ��������ʵ�Ļ���������֪ʶ������������������


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | v��NH3��=$\frac{2}{3}$v��H2O�� | B�� | v��O2��=$\frac{6}{5}$v��H2O�� | C�� | v��NH3��=$\frac{5}{4}$v��O2�� | D�� | v��O2��=$\frac{4}{5}$v��NO�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | K+ | B�� | Ba2+ | C�� | Zn2+ | D�� | Mg2+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Naʧ������CO2���Kʧ��Ҳ������CO2��� | |
| B�� | Al��Sֱ�ӻ��Ͽ��Եõ�Al2S3��Fe��Sֱ�ӻ�����ò���Fe2S3 | |
| C�� | ��SO2ͨ��BaCl2��Һ��û�а�ɫ�������ɣ���SO2ͨ��Ba��NO3��2��Һ��Ҳû�а�ɫ�������� | |
| D�� | CuSO4•5H2OͶ��ŨH2SO4�У�������ɫ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����ʵ�����MgCl2��Ba��OH��2��HC1��Һ��ϣ�Mg2++2OH-�TMg��OH��2�� | |
| B�� | NaHCO3��Һ�м���ϡHCl��CO32-+2H+�TCO2��+H2O | |
| C�� | AlCl3��Һ�м������ϡ��ˮ��Al3++4NH3•H2O�TAlO2-+4NH4++2H2O | |
| D�� | Cu����ϡHNO3��3Cu+8H++2NO3-�T3Cu2++2NO��+4H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | K+��Fe2+��NO3-��Cl-- | B�� | K+��Al3+��SO42-��Cl- | ||
| C�� | K+��NH4+��SO42-��Cl- | D�� | Na+��K+��Br-��AlO2- |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com