����⣺��1��SO
2����V
2O
5��Ӧ����V
2O
4��V
2O
5���뷴Ӧ������������������ԭΪV
2O
4���������������������
�ʴ�Ϊ��SO
2+V
2O
5?V
2O
4+SO
3��
��2�����ݹ�ʽn=c��v��I
2�����ʵ���Ϊn=0.0500mol/L��0.01L=5��10
-4mol�����ݷ�Ӧ��
SO
2 +I
2 +2H
2O=H
2SO
4+2HI
5��10
-4mol 5��10
-4mol
��״���µ����Ϊ5��10
-4mol��22.4L/mol=1.12��10
-2L��V���ɼ�������=100.0mL��������Ϊ��״��������������SO
2���������Ϊ
=0.112���ʴ�Ϊ��0.112��
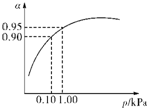
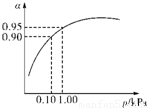
��3����ѹǿΪ0.1KPaʱ��SO
2��ת����Ϊ0.90��SO
2��ת�����Ѻܸߣ���ѹǿΪ1.0KPaʱ��SO
2��ת����Ϊ0.95��
��ʱ�����豸����Դ���кܸߵ�Ҫ��ѹǿ����������SO
2ת���ʵı仯�������ԣ������ϲ����㣬
�ʴ�Ϊ���ڳ�ѹʱ��SO
2��ת�����Ѻܸߣ�����ѹ������豸����Դ���нϸߵ�Ҫ�����ϲ����㣻
��4�����¶���ͬ�������Ϊ1L�������ܱ������У�SO
3�����ʵ�����Ϊ1mol����ѧƽ��״̬�Ĵﵽ�뻯ѧ��Ӧ;���أ�����ͬ�������£����淴Ӧ�����۴�����Ӧ��ʼ���Ǵ��淴Ӧ��ʼ�����ǴӼ��з�Ӧ�����������↑ʼ���ﵽ�Ļ�ѧƽ��״̬����ͬ�ģ�ƽ�������и�������ʵİٷֺ������ֲ��䣬���ǵ�Чƽ�⣮�����ܱ����������ֺ��¡����ݣ�����c��SO
3��=1.4 mol?L
-1�����������еķ�ӦΪ��Чƽ�⣮�����Ϊ1L������n��SO
3��=1.4 mol��
�� 2SO
2��g��+O
2��g��?2SO
3��g������H=-98.3kJ?mol
-1��
��ʼ����mol�� 2 1 0
�仯�� ��mol�� 1.4 0.7 1.4-98.3kJ��
=68.81KJ
ƽ���� ��mol�� 0.6 0.3
��a=68.81����
1=
=0.7��
���������ֺ��¡����ݣ��ͼ������з�ӦΪ��Чƽ�⣮��b=98.3kJ����2-1.4��/2=29.49kJ���÷�Ӧ����������ʼ����
2=
=0.3����
1+��
2=0.7+0.3=1��
�� 2SO
2��g��+O
2��g��?2SO
3��g������H=-98.3kJ?mol
-1������ckJ˵����Ӧ���淴Ӧ����ʼ��
��ʼ����mol�� m n p
�仯�� ��mol�� 0.2 0.1 1.4�£�1-12.5%��-1.4
ƽ���� ��mol�� m+0.2 n+0.1 1.4
P=1.4�£�1-12.5%��=1.6��c=98.3kJ����1.6-1.4��/2=9.83
b+c=29.49+9.83=39.32kJ��
�ʴ�Ϊ��1��1.6��39.32��

 ����������Ҫ����Ƿ��������ȣ��������Ʒ����������������裮
����������Ҫ����Ƿ��������ȣ��������Ʒ����������������裮


 ����������Ҫ����Ƿ��������ȣ��������Ʒ����������������裮
����������Ҫ����Ƿ��������ȣ��������Ʒ����������������裮