
(9��)
(1)3.6g H2O�����ʵ�����________������________mol H��
(2)�ڱ�״���£�4g H2��11.2 L O2��1mol H2O�У�����������������________������������________�����ѧʽ��
(3)�������Ķ�������������������ǵ����ʵ���֮��Ϊ________����������ԭ����֮��Ϊ________����������ԭ����֮��Ϊ________��
��4���������ʣ���H2O ��ʯī ��NH4NO3 ������ ��CH3COOH ����������������ʵ��� �����ڷǵ���ʵ��� ���ñ����д����
(1)0.2mol��0.4 (2)H2��H2O��(3)5��4��5��4��5��6��4���٢� ��
���������������1��3.6gH2O�����ʵ���Ϊ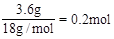 ������0.4molH��
������0.4molH��
��2����״���£�4gH2�����ʵ���Ϊ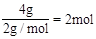 ��11.2LO2�����ʵ���Ϊ
��11.2LO2�����ʵ���Ϊ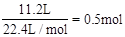 ������Ϊ16g��1molH2O������Ϊ18g����˺�������������H2������������H2O��
������Ϊ16g��1molH2O������Ϊ18g����˺�������������H2������������H2O��
��3���������Ķ�������������������ǵ����ʵ���֮��Ϊ5��4����������ԭ����֮��Ϊ5��4����������ԭ����֮��Ϊ5��6��
��4���������Ϊ��ˮ�����ᡣ�ǵ����Ϊ�����ǡ�
���㣺���ʵ����������ۺ�
����������ʺͷǵ���ʶ����ڻ�������ʻ�����Ȳ��ǵ����Ҳ���Ƿǵ���ʡ��������Ҫ��������κͽ���������ǵ������Ҫ�����л���ʹֵķǽ��������ǿ����ʰ���ǿ�ᡢǿ��ʹֵ����࣬������ʰ������ᡢ�����ˮ��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��9��)(1)�ڱ�״���£���6.72 L CH4���壻��3.01��1023�� HCl������ӣ���13.6 g H2S���壻��0.2 mol NH3�����ж����������������������ʵ����Ĺ�ϵ��С�����������(������������ű�ʾ)_______ ______________________________��
(2)���ξ��ᴿ��õ�NaCl��Һ���پ��������ᾧ����ɵþ��Ρ�
������������ʹ�õ�������������̨(����Ȧ)�⣬����Ҫ����������Ϊ��д�����֣�_________________��
�ڸ�ͬѧ�����þ��������Һ��������һʵ�飺ʵ������Ҫ��60 mL 2 mol/L��NaCl��Һ�����ƹ�������������ƽ��ȡ�ľ�������Ϊ________����ͬѧ�����η�����ƽ�������г����ú����Ƴ���Һ�������Һ��Ũ��________2mol��L��1(�����������������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ������ʡ��ɽ�����ѧ��һ��ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
��9��)(1)�ڱ�״���£���6.72 L CH4���壻��3.01��1023�� HCl������ӣ���13.6 g H2S���壻��0.2 mol NH3�����ж����������������������ʵ����Ĺ�ϵ��С�����������(������������ű�ʾ)_______ ______________________________��
(2)���ξ��ᴿ��õ�NaCl��Һ���پ��������ᾧ����ɵþ��Ρ�
������������ʹ�õ�������������̨(����Ȧ)�⣬����Ҫ����������Ϊ��д�����֣�_________________��
�ڸ�ͬѧ�����þ��������Һ��������һʵ�飺ʵ������Ҫ��60 mL 2 mol/L��NaCl��Һ�����ƹ�������������ƽ��ȡ�ľ�������Ϊ________����ͬѧ�����η�����ƽ�������г����ú����Ƴ���Һ�������Һ��Ũ��________2 mol��L��1(�����������������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014������ʡ��һ��ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
��9��)(1)�ڱ�״���£���6.72 L CH4���壻��3.01��1023�� HCl������ӣ���13.6 g H2S���壻��0.2 mol NH3�����ж����������������������ʵ����Ĺ�ϵ��С�����������(������������ű�ʾ)_______ ______________________________��
(2)���ξ��ᴿ��õ�NaCl��Һ���پ��������ᾧ����ɵþ��Ρ�
������������ʹ�õ�������������̨(����Ȧ)�⣬����Ҫ����������Ϊ��д�����֣�_________________��
�ڸ�ͬѧ�����þ��������Һ��������һʵ�飺ʵ������Ҫ��60 mL 2 mol/L��NaCl��Һ�����ƹ�������������ƽ��ȡ�ľ�������Ϊ________����ͬѧ�����η�����ƽ�������г����ú����Ƴ���Һ�������Һ��Ũ��________2 mol��L��1(�����������������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�����������ѧ�߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ�ʵ����
(12��)ijͬѧ�Ա�ȩ��������Ӧ�������о���
I.����������Һ��
(1)����������Һʱ���Ѱ�ˮ������������Һ�IJ����ؼ��ǣ�
(2)����������Һ�������漰�����ӷ���ʽΪ
II.̽��������Ӧ�����ʵ������������ʵ���������±�����
|
ʵ�����
ʵ����� |
������Һ ����/mL |
��ȩ����/�� |
ˮԡ�¶�/0C |
��Ӧ���Һ ��pH |
�������� ��ʱ��/min |
|
1 |
1 |
3 |
65 |
11 |
5 |
|
2 |
1 |
3 |
45 |
11 |
6.5 |
|
3 |
1 |
5 |
65 |
11 |
4 |
|
4 |
1 |
3 |
30 |
11 |
9 |
|
5 |
1 |
3 |
50 |
11 |
6 |
|
6 |
1 |
5 |
80 |
11 |
3 |
(3)��ֻ����ʵ��1��ʵ��3����̽��Ŀ����
(4)��������Һ����Ϊ1 mL����ȩ������Ϊ3�Σ�ˮԡ�¶�Ϊ400C����Ӧ���ҺpHΪ11ʱ������������ʱ�䷶ΧӦ����
(5)����Ҫ̽��������Һ�������Գ�������������Ӱ�죬��ν���ʵ�飿
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(12��)ijͬѧ�Ա�ȩ��������Ӧ�������о���
I.����������Һ��
(1)����������Һʱ���Ѱ�ˮ������������Һ�IJ����ؼ��ǣ�
(2)����������Һ�������漰�����ӷ���ʽΪ
II.̽��������Ӧ�����ʵ������������ʵ���������±�����
| ʵ�����
ʵ����� | ������Һ ����/mL | ��ȩ����/�� | ˮԡ�¶�/0C | ��Ӧ���Һ ��pH | �������� ��ʱ��/min |
| 1 | 1 | 3 | 65 | 11 | 5 |
| 2 | 1 | 3 | 45 | 11 | 6.5 |
| 3 | 1 | 5 | 65 | 11 | 4 |
| 4 | 1 | 3 | 30 | 11 | 9 |
| 5 | 1 | 3 | 50 | 11 | 6 |
| 6 | 1 | 5 | 80 | 11 | 3 |
(3)��ֻ����ʵ��1��ʵ��3����̽��Ŀ����
(4)��������Һ����Ϊ1 mL����ȩ������Ϊ3�Σ�ˮԡ�¶�Ϊ400C����Ӧ���ҺpHΪ11ʱ������������ʱ�䷶ΧӦ����
(5)����Ҫ̽��������Һ�������Գ�������������Ӱ�죬��ν���ʵ�飿
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com