
��һ���¶���,���������������淴ӦA(��)+3B(��)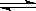 2C(��)+2D(��)�ﵽƽ��ı�־���� ( )
2C(��)+2D(��)�ﵽƽ��ı�־���� ( )
��C������ ������C�ķֽ�������� �ڵ�λʱ��������amolA,ͬʱ����3amolB
��A��B��C��Ũ�Ȳ��ٱ仯 ��A��B��C�İٷֺ������ڱ仯
�ݻ���������ѹǿ���ٱ仯 �����������ʵ������ٱ仯
�ߵ�λʱ��������amolA,ͬʱ���� 3amolB ��A��B��C��D�ķ�����֮��Ϊ1:3:2:2
A.�٢ۢܢݢޢߢ� B.�ڢݢ� C.�٢ۢܢݢޢ� D.�ڢݢޢ�

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �¶�/�� | 1 000 | 1 150 | 1 300 |
| ƽ�ⳣ�� | 64.0 | 50.7 | 42.9 |
| c3(CO3) |
| c3(CO) |
| c3(CO3) |
| c3(CO) |
| ������� | ��ʼʱ���������ʵ���/mol | �ﵽƽ���ʱ��/min | ��ƽ��ʱ��ϵ�����ı仯/kJ | ||||
| CO | H2O | CO2 | H2 | ||||
| �� | 1 | 4 | 0 | 0 | t1 | �ų�������32.8kJ | |
| �� | 2 | 8 | 0 | 0 | t2 | �ų�������Q | |
| 4 |
| Vt1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012�����ʡ�����и߶���ѧ�ڵ�һ���¿���ѧ�Ծ� ���ͣ�ѡ����
��һ���¶���,�����������ǿ��淴Ӧ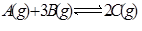 �ﵽƽ��ı�־���ǣ� ��
�ﵽƽ��ı�־���ǣ� ��
��1��C������������C�ķֽ�������ȣ���2����λʱ������amol A��ͬʱ����3amol B����3��A��B��C��Ũ�Ȳ��ٱ仯����4��2v����B��=3v����C������5������������ѹǿ���ٱ仯����6�������������ʵ������ٱ仯����7����������ƽ����Է����������ֲ���ʱ����8��A��B��C�ķ�����Ŀ��Ϊ1:3:2��
A. ��2����8�� B. ��7����4�� C. ��1����3�� D. ��5����6��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ���¶���,�����������ǿ��淴Ӧ![]() �ﵽƽ��ı�־���ǣ� ��
�ﵽƽ��ı�־���ǣ� ��
��1��C������������C�ķֽ�������ȣ���2����λʱ������amol A��ͬʱ����3amol B����3��A��B��C��Ũ�Ȳ��ٱ仯����4��2v����B��=3v����C������5������������ѹǿ���ٱ仯����6�������������ʵ������ٱ仯����7����������ƽ����Է����������ֲ���ʱ����8��A��B��C�ķ�����Ŀ��Ϊ1:3:2��
A. ��2����8�� B.��7����4�� C. ��1����3�� D. ��5����6��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012�����ʡ�����е���ʵ����и߶���ѧ�ڵ�һ���¿���ѧ�Ծ� ���ͣ���ѡ��
��һ���¶���,�����������ǿ��淴Ӧ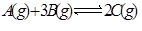 �ﵽƽ��ı�־���ǣ� ��
�ﵽƽ��ı�־���ǣ� ��
��1��C������������C�ķֽ�������ȣ���2����λʱ������amol A��ͬʱ����3amol B����3��A��B��C��Ũ�Ȳ��ٱ仯����4��2v����B��=3v����C������5������������ѹǿ���ٱ仯����6�������������ʵ������ٱ仯����7����������ƽ����Է����������ֲ���ʱ����8��A��B��C�ķ�����Ŀ��Ϊ1:3:2��
A. ��2����8�� B. ��7����4�� C. ��1����3�� D. ��5����6��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com