
 2+��
2+�� ���� ��1����Mnԭ�Ӻ��������Ϊ25�����ڵ������ڢ�B�壻Cuԭ�Ӻ��������Ϊ29�������������ԭ����д��������Ų�ʽ��
�ڼ���Cԭ�Ӽ۲���Ӷ������µ��Ӷ���ȷ���ռ乹�ͣ�
��2����CO��N2��Ϊ�ȵ����壬���߽ṹ���ƣ�CO������Cԭ������ԭ��֮���γ�3�Թ��õ��Ӷԣ�
��CO2 ������Cԭ������2���Ҽ���û�йµ��Ӷԣ��ӻ������ĿΪ2��
��HCHO�����к���2��C-H����1��C=O˫���������к���3���Ҽ���
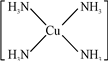
��3��[Cu ��NH3��4]2+����Cu2+��4��NH3������
��� �⣺��1����Mnԭ�Ӻ��������Ϊ25�����ڵ������ڢ�B�壬�۵����Ų�ʽΪ3d54s2���ʴ�Ϊ��3d54s2��
��CO32-��Cԭ�ӹµ��Ӷ���=$\frac{1}{2}$��4+2-2��3��=0���۲���Ӷ���=3+0=3���ռ乹��Ϊƽ�������Σ��ʴ�Ϊ��ƽ�������Σ�
��2����CO��N2��Ϊ�ȵ����壬���߽ṹ���ƣ�CO������Cԭ������ԭ��֮���γ�3�Թ��õ��Ӷԣ�CO�ṹʽΪC��O���ʴ�Ϊ��C��O��
��CO2 ������Cԭ������2���Ҽ���û�йµ��Ӷԣ��ӻ������ĿΪ2��Cԭ�Ӳ�ȡsp�ӻ����ʴ�Ϊ��sp��
��HCHO�����к���2��C-H����1��C=O˫���������к���3���Ҽ���1mol��ȩ��HCHO�������к��еĦҼ���ĿΪ3��6.02��1023����3 NA����
�ʴ�Ϊ��3��6.02��1023����3 NA����
��3��[Cu��NH3��4]2+����Cu2+��4��NH3�γ���λ������[Cu��NH3��4]2+�Ľṹ����ʾ��ͼ��ʾΪ�� 2+��
2+��
�ʴ�Ϊ 2+��
2+��
���� �����Ƕ����ʽṹ�����ʵĿ��飬�漰��������Ų����ӻ���ʽ��ռ乹���жϡ��ȵ����塢��ѧ���������ȣ�ע��Ի���֪ʶ���������գ�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ѧ������ʹ�������ϣ�Ҳ����ʹԭ������ | |
| B�� | �ǽ���Ԫ�ص�ԭ��֮��ֻ���γɹ��ۼ� | |
| C�� | ��ѧ��Ӧ�����У���Ӧ������ڵĻ�ѧ�����ѣ���������л�ѧ���γ� | |
| D�� | ��ѧ����һ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | C��Mg��Li | B�� | Li��C��Mg | C�� | C��Si��Mg | D�� | C��O��Mg |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ʵ����� | ʵ��Ŀ�� |
| A | ���ȵ�NaOH��Һ�е��뱥��FeCl3��Һ | �Ʊ�Fe��OH��3���� |
| B | ��SO2ͨ��KMnO4��Һ | ��֤SO2��Ư���� |
| C | ������Fe3+��MgCl2��Һ�м�������MgCO3��ĩ�����ȡ����貢���� | ��ȥMgCl2��Һ��������Fe3+ |
| D | ��0.1mol•L-1��Na2SO4��Һ����BaCl2��Һ�������г����������ٵμ�0.1mol•L-1��Na2CO3��Һ | �Ƚ�BaCO3��BaSO4�ܶȻ��Ĵ�С |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
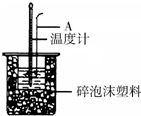 ������ͼװ�òⶨ�к��ȵ�ʵ�鲽�����£�
������ͼװ�òⶨ�к��ȵ�ʵ�鲽�����£�| �¶� ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶� t2/�� | �¶Ȳ�ƽ��ֵ ��t2-t1��/�� | ||
| H2SO4 | NaOH | ƽ��ֵ | |||
| 1 | 25.0 | 25.2 | 28.5 | ||
| 2 | 24.9 | 25.1 | 28.3 | ||
| 3 | 25.5 | 26.5 | 31.8 | ||
| 4 | 25.6 | 25.4 | 29.0 | ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ʧȥ�ĵ���������� | |
| B�� | ��������С�ĵ���������� | |
| C�� | 2p���������������2s����������� | |
| D�� | ���������������˶��ĵ���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CO2��H2O2 | B�� | C2H4��CH4 | C�� | C60��C2H4 | D�� | NH3��HCl |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ԭ������A��B | B�� | ��������A-��B2+ | C�� | ���Ӱ뾶A-��B2+ | D�� | ԭ�Ӱ뾶A��B |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ۡ���ά�ء���֬�����ڸ߷��ӻ����� | |
| B�� | ����ζ�����ʲ�һ���������� | |
| C�� | ��Ȼ������ˮ������ղ����Ϊ������ | |
| D�� | ��֬ˮ��õ��Ĵ��DZ����� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com