
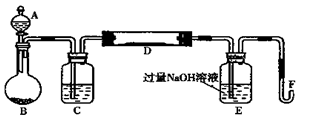
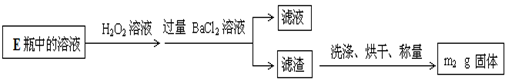

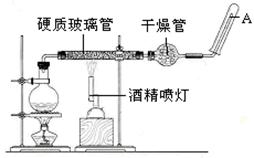
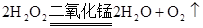 ������C֮����������˼���װ������Cװ�õ������Ǹ������壬����C�зŵ���Ũ������ڷ�Ӧ���ɶ��������������Eƿ�����������շ�Ӧ���ɵĶ�������ʹ��ȫ��ת��Ϊ����������ӣ������ڸ�������������ӵ���������Ʒ����Ԫ�ص�������
������C֮����������˼���װ������Cװ�õ������Ǹ������壬����C�зŵ���Ũ������ڷ�Ӧ���ɶ��������������Eƿ�����������շ�Ӧ���ɵĶ�������ʹ��ȫ��ת��Ϊ����������ӣ������ڸ�������������ӵ���������Ʒ����Ԫ�ص�������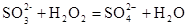
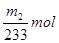 ���������غ���������Ԫ������Ϊ��
���������غ���������Ԫ������Ϊ�� ������Ʒ����Ԫ�ص���������Ϊ
������Ʒ����Ԫ�ص���������Ϊ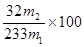 % ����Ԫ�ص��������������Ը���������������ӷ�Ӧ�ķ���ʽΪ
% ����Ԫ�ص��������������Ը���������������ӷ�Ӧ�ķ���ʽΪ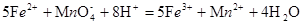 �ζ����ĵĸ������
�ζ����ĵĸ������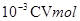 �����ݷ���ʽ���Լ����25MLϡ��Һ���������ӵ����ʵ���Ϊ
�����ݷ���ʽ���Լ����25MLϡ��Һ���������ӵ����ʵ���Ϊ ������250 ML��Һ�й����������ӵ����ʵ���Ϊ
������250 ML��Һ�й����������ӵ����ʵ���Ϊ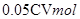 ���ݴ˿ɵó�ԭ��Ʒ����Ԫ�ص�����Ϊ
���ݴ˿ɵó�ԭ��Ʒ����Ԫ�ص�����Ϊ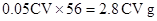 ��Ʒ����Ԫ�ص���������Ϊ
��Ʒ����Ԫ�ص���������Ϊ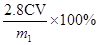


 ��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

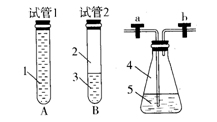
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��KI������Һ��ͨ��Cl2����Һ����ԭ���ǵ�������Cl2������ɫ��Ӧ |
| B��Ũ�����ڹ��������±��������Ũ����ȶ���������ɫ����������Ũ���� |
| C��ij��Һ�м��������ữ���Ȼ�����Һ���а�ɫ��������˵������Һ�к���SO42- |
| D�������£�Ũ����ɴ��������ʻ�����������˵����������������Ũ�����Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
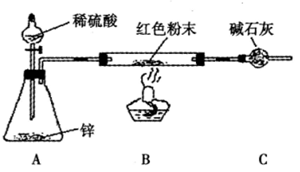
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
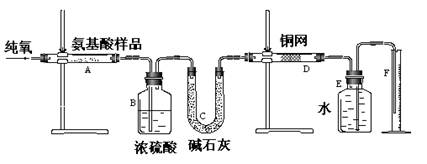
| A�����ɶ�����̼��������� | B������ˮ������ |
| C��ͨ����������� | D�����������Է������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ѡ�� | ʵ������ | ʵ����쳣��� | ԭ����� |
| A | �Ʊ�Fe(OH)2 | �۲첻����ɫ���� | ����ԭ���е�Fe2����������δ�������� |
| B | �����ᾧ | ���������� | �ƾ��Ƶ�о�����ȵ�������ײ���������ʣ����Һ��ʱ�������� |
| C | ����ˮ��CCl4 | ��Һ©���������²�Һ���������� | û��װ©�������Ӱε��������ϰ�����©���ڲ����С��û�ж��� |
| D | ��ȼ����ȥCO2�е�CO���� | ����ȼ | CO���Ż��ϸ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
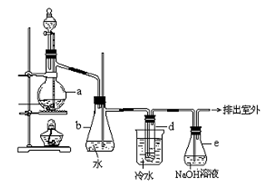
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
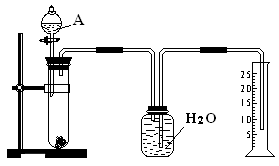
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com