
 �����Ʋ�1mol NH4BF4��������泥��к���2NA����λ����
�����Ʋ�1mol NH4BF4��������泥��к���2NA����λ����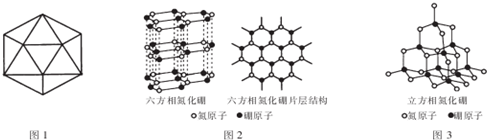
���� ��1����ͼ��Ԫ���غ��д����B2O3�Ʊ�BF3�ķ���ʽ�����ݼ۲���ӶԻ�������ȷ�����ͺ��ӻ���ʽ��
��2��ͬһ����Ԫ�أ�Ԫ�صĵ�һ����������ԭ�����������������IIA�塢��VA��Ԫ�ص�һ�����ܴ���������Ԫ�أ��ݴ��жϵ�һ�����ܴ�С˳��
��3����[Al��OH��4]-��Al��ȡsp3�ӻ������������ĸ��չ������O�ṩ�ŶԵ����γ�����һ��NH4BF4��Nԭ�Ӻ�����һ��Hԭ��֮�������λ����Bԭ�Ӻ�����һ��Fԭ��֮�����һ����λ�������Ժ���2����λ�����ݴ˼��㣻
��4������ԭ����ɵ�����ʮ����ṹ�У�ÿ5���湲��һ�����㣬ÿ���ȱ�������ӵ�еĶ���Ϊ��$\frac{1}{5}$��3=$\frac{3}{5}$��20���ȱ�������ӵ�еĶ���Ϊ��$\frac{3}{5}$��20=12��ÿ2���湲��һ��B-B����
��5����a����ͼ��֪�����൪������������״�ṹ��Ϊԭ�Ӿ��壻
b���ǽ���Ԫ��֮�����γɹ��ۼ���
c�������൪����Ϊ��״�ṹ�����Ϊ���Ӽ���������������С��
d�������൪�����ЦҼ������ڦм���
�������൪���������һ����ԭ�������ڵ�ԭ���γ�3�����۵����������ʵIJ�״�ṹ�в����������ƶ��ĵ��ӣ�
�۵�������̼Ԫ�صĵ������ƣ���Ͻ��ʯ�Ľṹ�����жϣ������൪�������У�ÿ����ԭ������12����Ԫ�����ڵؿ��ڲ��������Խ���ѹǿԽ���¶�Խ�ߣ�
��� �⣺��1��B2O3��CaF2��H2SO4��Ӧ������BF3��ͬʱ��Ӧ�ò�������ƺ�ˮ������ʽΪ��B2O3+3CaF2+3H2SO4=2BF3��+3CaSO4+3H2O��BF3���ӵ�����ԭ��Bԭ���Ϻ���3���� ��������ԭ���ϵŵ��Ӷ���=$\frac{1}{2}$��a-xb��=$\frac{1}{2}$��0-3��1��=0������ԭ��Bԭ�ӵļ۲���Ӷ���Ϊ3������sp2�ӻ�������ԭ����û�й¶Ե��ӣ�������ռ乹�;���ƽ�������Σ�������120�㣬BF3����Ϊƽ�������Σ�
�ʴ�Ϊ��B2O3+3CaF2+3H2SO4=2BF3��+3CaSO4+3H2O��sp2��ƽ���������Σ�
��2��ͬһ����Ԫ�أ�Ԫ�صĵ�һ����������ԭ�����������������IIA�塢��VA��Ԫ�ص�һ�����ܴ���������Ԫ�أ�B��N��O��FԪ�ش���ͬһ������ԭ������������N���ڵ�VA�壬���Ե�һ������N��O��B�ĵ�һ��������С����һ�������ɴ�С��˳���ǣ�F��N��O��B��
�ʴ�Ϊ��F��N��O��B��
��3����[Al��OH��4]-��Al��ȡsp3�ӻ������������ĸ��չ������O�ṩ�ŶԵ����γ�������ṹʽΪ ��һ��NH4BF4��Nԭ�Ӻ�����һ��Hԭ��֮�������λ����Bԭ�Ӻ�����һ��Fԭ��֮�����һ����λ�������Ժ���2����λ������1mol NH4BF4����2mol��λ������2NA����λ����
��һ��NH4BF4��Nԭ�Ӻ�����һ��Hԭ��֮�������λ����Bԭ�Ӻ�����һ��Fԭ��֮�����һ����λ�������Ժ���2����λ������1mol NH4BF4����2mol��λ������2NA����λ����
�ʴ�Ϊ�� ��2NA��
��2NA��
��4������ԭ����ɵ�����ʮ����ṹ�У�ÿ5���湲��һ�����㣬ÿ����ӵ���������ģ�$\frac{1}{5}$��3=$\frac{3}{5}$��20���ȱ�������ӵ�еĶ���Ϊ��$\frac{3}{5}$��20=12��ÿ2���湲��һ��B-B����ÿ����ӵ�����B-B����$\frac{1}{2}$��ÿ���ȱ�������ռ�е�B-B��Ϊ��$\frac{1}{2}$��3=$\frac{3}{2}$��20���ȱ�������ӵ�е�B-B��Ϊ��$\frac{3}{2}$��20=30��
�ʴ�Ϊ��30��
��5����a�������൪����Ϊ�ռ���״�ṹ�������ڷ��ӣ�Ϊԭ�Ӿ��壬��a����
b���ǽ���Ԫ��֮�����γɹ��ۼ�������Nԭ�Ӻ�Bԭ��֮����ڹ��ۼ�����b��ȷ��
c�������൪������Ϊ��״�ṹ�����Ӽ���������������С���������ʵ�������c��ȷ��
d�������൪����Nԭ�Ӻ�Bԭ��֮����ڹ��۵��������Ըû������к��ЦҼ������ڦм�����d����
��ѡad��
�������൪���������һ����ԭ�������ڵ�ԭ���γ�3�����۵����������൪��������B-N��������ԭ����֮��Ϊ3��1�������ʵIJ�״�ṹ�в����������ƶ��ĵ��ӣ����Բ����磬
�ʴ�Ϊ��3��1�����������������������ƶ��ĵ��ӣ�
�۵���������ʯ�Ľṹ���ƣ������൪�������У�ÿ����ԭ������12����Ԫ�����ڵؿ��ڲ��������Խ���ѹǿԽ���¶�Խ�ߣ��������֪��ʵ�����������൪����ϳ������൪������Ҫ������Ӧ�Ǹ��¸�ѹ��
�ʴ�Ϊ��12�����¡���ѹ��
���� ���⿼�������ʽṹ�����ʣ��漰��һ�����ܡ�ԭ�ӵ��ӻ���ʽ������ļ����֪ʶ�㣬������ѧ���ķ��������ͼ��������Ŀ��飬��Щ֪ʶ�㶼�Ǹ߿��ȵ㣬ע��۲���ӶԻ�������ȷ��ԭ���ӻ���ʽ�����ӿռ乹�͡������ļ��㣬��Ŀ�Ѷ��еȣ�


 ����Ӣ��ϵ�д�
����Ӣ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  �ⶨ�к��� | B�� |  ��Ӧ���� | C�� |  ��ͭ�ĵ�⾫�� | D�� |  ���ʳ��ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
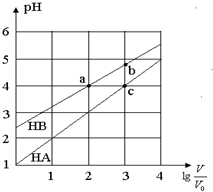 �����£�Ũ�Ⱦ�Ϊ0.10mol/L�������ΪV0��HA��HB��Һ���ֱ��ˮϡ�������V��pH��lg$\frac{V}{{V}_{0}}$�ı仯��ͼ��ʾ������������ȷ���ǣ�������
�����£�Ũ�Ⱦ�Ϊ0.10mol/L�������ΪV0��HA��HB��Һ���ֱ��ˮϡ�������V��pH��lg$\frac{V}{{V}_{0}}$�ı仯��ͼ��ʾ������������ȷ���ǣ�������| A�� | ���¶���HB�ĵ���ƽ�ⳣ��Լ����1.11��10-5 | |
| B�� | ��lg$\frac{V}{{V}_{0}}$=3ʱ��������Һͬʱ�����¶ȣ���$\frac{c��{B}^{-}��}{c��{A}^{-}��}$��С | |
| C�� | ��ͬ������NaA��Һ��pH����NaB��Һ��pH | |
| D�� | ��Һ��ˮ�ĵ���̶ȣ�a=c��b |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
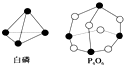 ��ѧ��Ӧ����Ϊ�ɼ����Ѻ��¼��γɵĹ��̣���ѧ���ļ������γɣ����1mol��ѧ��ʱ�ͷţ������գ�����������֪����P4O6�ķ��ӽṹ��ͼ��ʾ�����ṩ���»�ѧ���ļ��ܣ�kJ/mol����P-P��198��P-O��360��O=O��498�����ڷ�ӦP4�����ף�+3O2=P4O6����2mol��������������ַ�Ӧ�������仯Ϊ��������
��ѧ��Ӧ����Ϊ�ɼ����Ѻ��¼��γɵĹ��̣���ѧ���ļ������γɣ����1mol��ѧ��ʱ�ͷţ������գ�����������֪����P4O6�ķ��ӽṹ��ͼ��ʾ�����ṩ���»�ѧ���ļ��ܣ�kJ/mol����P-P��198��P-O��360��O=O��498�����ڷ�ӦP4�����ף�+3O2=P4O6����2mol��������������ַ�Ӧ�������仯Ϊ��������| A�� | ����1638 kJ | B�� | ����1638 kJ | C�� | ����3276 kJ | D�� | ����3276 kJ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | K | B�� | Ca | C�� | I | D�� | Ne |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ������±����ش��������⣺�����ݾ���25��ʱ�ⶨ��
������±����ش��������⣺�����ݾ���25��ʱ�ⶨ��| ��ѧʽ | CH3COOH | H2CO3 | HClO | Cu��OH��2 |
| ��س��� | Ka=1.8��10-5 | Ka1=4.3��10-7 Ka2=5.6��10-11 | Ka=3.0��10-8 | Ksp=2��10-20 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | H2O2 | B�� | NaOH | C�� | CaO | D�� | SO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ClO2������һ����ˮ�����ȷ����й㷺Ӧ�õĸ�Ч��ȫ���������ҹ���2000��������ClO2��������������ˮ����������
ClO2������һ����ˮ�����ȷ����й㷺Ӧ�õĸ�Ч��ȫ���������ҹ���2000��������ClO2��������������ˮ�����������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com