
̫��������ϵ�м�Ϊ��ͨ��һ�ź��ǣ����ҵ��Ⱥ˷�Ӧʹ֮���ϵ��������������̫��������������ּ��������Ⲩ�Σ��ɼ��Ⲩ�κͺ���Ⲩ�Σ����ǵ������ֱ�ռ�ܷ�������9%��44%��47%������Ĵ������У���������ijɷ�Ϊ��������벵ȣ�ռ����������99.96%���ɱ�����ɷ���Ҫ��CO2��ˮ���ͳ����ȣ���Щ����ĺ�����С�����Դ�������״����Ӱ�켫����������һ����ָ�߶��������10~15Km�Ĵ����㣬���г�����Ũ�Ⱥܵͣ������ۺϳɱ�״���������ۻ����Ҳ����0.3cm���京����С�����Ե�������������Ӱ��ܴ�
��1������ѹǿP������߶�Z��m�����������С�����鹫ʽΪPZ=![]() ������P0Ϊ�ر�����ѹֵ��e=2.718���������㼯����24km�ĸ߿մ������ڸø߶ȴ����¶�Ϊ��50�档�Թ��������ĺ�ȡ�
������P0Ϊ�ر�����ѹֵ��e=2.718���������㼯����24km�ĸ߿մ������ڸø߶ȴ����¶�Ϊ��50�档�Թ��������ĺ�ȡ�
��2����Ҫ˵��������Ե�������Ȧ�����塣
![]() ��3����������������Լ����µķ�Ӧ���̣��߲�����е�һ����Ҫ�Ĺ⻯ѧ��Ӧ������������̫�������в�����<0.24��m�Ĺ��ӣ����Ϊ��ԭ�ӣ���O2+h�ͣ���<0.24��m�� O+O���ɴ˿��γ�һϵ�еķ�Ӧ����������Ҫ������ԭ�Ӻ��������ڵ����壨M���IJ������γɳ������䷴Ӧ����ʽΪ �������M��Ҫ�������ӻ��ӣ��������ڷ�Ӧ������ͬʱ���������غ�Ͷ����غ�������ġ������ڦ�<0.18��m�ķ��������£������������Ӻ���ԭ�ӣ��䷴Ӧ����ʽΪ ��
��3����������������Լ����µķ�Ӧ���̣��߲�����е�һ����Ҫ�Ĺ⻯ѧ��Ӧ������������̫�������в�����<0.24��m�Ĺ��ӣ����Ϊ��ԭ�ӣ���O2+h�ͣ���<0.24��m�� O+O���ɴ˿��γ�һϵ�еķ�Ӧ����������Ҫ������ԭ�Ӻ��������ڵ����壨M���IJ������γɳ������䷴Ӧ����ʽΪ �������M��Ҫ�������ӻ��ӣ��������ڷ�Ӧ������ͬʱ���������غ�Ͷ����غ�������ġ������ڦ�<0.18��m�ķ��������£������������Ӻ���ԭ�ӣ��䷴Ӧ����ʽΪ ��
��4������Ļ��������һЩ���壬�ܶԴ����еij������ƻ����á�ʹ�京�����٣���������غ����ֱ����в���������������档��Щ�������������ϼ��������������س��ֳ����ն��ı������������Ǽ���Ĺ�ע����Ҫ˵��������������Щ����Դ��������������ƻ����á�
��1��5cm
��2�������������߶��������棬�ɴٽ������ĸƻ���Ԥ�����Ͳ����������������Ŀ��������������������������ƻ����ﵰ�ͻ������ʣ����ϸ����������ʹƤ��������ߡ�ɫ�س�����Ƥ�������������ӣ����˺��۾��������ۼ��Խ�Ĥ�ס������ϡ���ʧ��������������������������ã����赲�������������߷��䵽�ر����Ե���������������ã���ˣ�������Ĵ��ڶԵ��������������Ҫ��
![]()
![]() ��3��O2+O+M O3+M O3+ h�ͣ���<0.18��m�� O2+O
��3��O2+O+M O3+M O3+ h�ͣ���<0.18��m�� O2+O
��4�������в��������壺�������ɻ��ŷŴ�����NO��һЩũҩ��ɱ������ܲ���N2O����Ϊ�����������ϵͳ�еĹ㷺ʹ�õķ��ﰺ����Щ��������ͷ��ﰺ�ȶԳ����㶼�����ƻ����á�
��������˳�������й�֪ʶ�������˳��������ɺ����ġ�����������ȵ���Ϣ��Ҫ��ѧ���ܹ�����ʾ��Ϣ����ȡ���������ص���Ϣ���ų���һЩ�����������⣬��Ҫ��ѧ���ܹ��Ը���������м��Ӷ����п�ѧ�Ĺ��㡣����Ŀ�ṩ�Ĺ�ʽ��PZ=![]() �������Z=24km�߿մ�����ѹPZ=
�������Z=24km�߿մ�����ѹPZ=![]() =
= ![]() = P0�� e-3=0.05P0������̬����
= P0�� e-3=0.05P0������̬����![]() ������
������![]() ����ΪD0��D<<Z�����У�
����ΪD0��D<<Z�����У�![]() ���ɴ˿ɵã�D=
���ɴ˿ɵã�D=![]() ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
���û�ѧ��Ӧԭ���о������������е�ʵ���������ʮ����Ҫ�����壺
�������������ϳɰ��ǻ�ѧ��ҵ�м�Ϊ��Ҫ�ķ�Ӧ�����Ȼ�ѧ����ʽ�ɱ�ʾΪ��N2(g)��3H2(g) 2NH3(g)����H����92 kJ��mol��1����ش��������⣺
(1)ȡ1 mol N2(g)��3 mol H2(g)����һ�ܱ������У��ڴ�������ʱ���з�Ӧ����÷�Ӧ�ų��������ߣߣߣߣ�92 kJ(����ڡ������ڡ���С�ڡ�)��ԭ���ǣߣߣߣߣߣߣߣ��������������H������(������С�����䡱)��
(2)��֪���ֱ��ƻ�1 mol N��N����1 mol H��H����Ҫ���յ�����Ϊ��946 kJ��436 kJ�����ƻ�1 mol N��H����Ҫ���յ�����Ϊ�ߣߣߣߣߣ�kJ��
(3)N2H4����Ϊ��NH3�����е�H����NH2ȡ���IJ������������N2H4(g)Ϊȼ�ϣ�NO2Ϊ����������N2��H2O(g)��
��֪��N2(g)��2O2(g)===2NO2(g) ��H1����67��7 kJ��mol��1
N2H4(g)��O2(g)===N2(g)��2H2O(g) ��H2����534 kJ��mol��1��
��1 mol N2H4��ȫ��Ӧ���Ȼ�ѧ����ʽΪ ��
����ijǦ���ص�����������DZ�ĥ��������ͼװ�����ʵ�飬ʶ�����Ǧ���ص���������
(1)��A��E��B��F����B�缫���� ����ӦʽΪ ����˵��FΪ������
��2����Ǧ���ع���ʱ���ŵ磩����E���ڵ缫�ĵ缫��ӦʽΪ�� �����ʱ�ü�����ӵ�Դ�� ��������
��3�����øõ�ص��Cu(NO3)2��Һ�����ⷽ��ʽΪ
����0.2mol���ӷ���ת�ƣ����������ĵ�PbO2�����ʵ����� ��Ҫ��CuSO4��Һ�ָ�ԭ���������������� ,����Ϊ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ɽ��ʡΫ���и߶�������ҵ��ѧ��һ���Ծ� ���ͣ������
���û�ѧ��Ӧԭ���о������������е�ʵ���������ʮ����Ҫ�����壺
�������������ϳɰ��ǻ�ѧ��ҵ�м�Ϊ��Ҫ�ķ�Ӧ�����Ȼ�ѧ����ʽ�ɱ�ʾΪ��N2(g)��3H2(g)  2NH3(g)����H����92 kJ��mol��1����ش��������⣺
2NH3(g)����H����92 kJ��mol��1����ش��������⣺
(1)ȡ1 mol N2(g)��3 mol H2(g)����һ�ܱ������У��ڴ�������ʱ���з�Ӧ����÷�Ӧ�ų��������ߣߣߣߣ�92 kJ(����ڡ������ڡ���С�ڡ�)��ԭ���ǣߣߣߣߣߣߣߣ��������������H������(������С�����䡱)��
(2)��֪���ֱ��ƻ�1 mol N��N����1 mol H��H����Ҫ���յ�����Ϊ��946 kJ��436 kJ�����ƻ�1 mol N��H����Ҫ���յ�����Ϊ�ߣߣߣߣߣ�kJ��
(3)N2H4����Ϊ��NH3�����е�H����NH2ȡ���IJ������������N2H4(g)Ϊȼ�ϣ�NO2Ϊ����������N2��H2O(g)��
��֪��N2(g)��2O2(g)===2NO2(g) ��H1����67��7 kJ��mol��1
N2H4(g)��O2(g)===N2(g)��2H2O(g) ��H2����534 kJ��mol��1��
��1 mol N2H4��ȫ��Ӧ���Ȼ�ѧ����ʽΪ ��
����ijǦ���ص�����������DZ�ĥ��������ͼװ�����ʵ�飬ʶ�����Ǧ���ص���������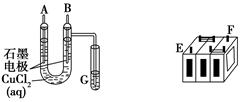
(1)��A��E��B��F����B�缫���� ����ӦʽΪ ����˵��FΪ������
��2����Ǧ���ع���ʱ���ŵ磩����E���ڵ缫�ĵ缫��ӦʽΪ�� �����ʱ�ü�����ӵ�Դ�� ��������
��3�����øõ�ص��Cu(NO3)2 ��Һ�����ⷽ��ʽΪ
����0.2mol���ӷ���ת�ƣ����������ĵ�PbO2�����ʵ����� ��Ҫ��CuSO4��Һ�ָ�ԭ���������������� ,����Ϊ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ɽ��ʡΫ���и߶�������ҵ��ѧ��һ���Ծ� ���ͣ������
���û�ѧ��Ӧԭ���о������������е�ʵ���������ʮ����Ҫ�����壺
�������������ϳɰ��ǻ�ѧ��ҵ�м�Ϊ��Ҫ�ķ�Ӧ�����Ȼ�ѧ����ʽ�ɱ�ʾΪ��N2(g)��3H2(g)  2NH3(g)����H����92 kJ��mol��1����ش��������⣺
2NH3(g)����H����92 kJ��mol��1����ش��������⣺
(1)ȡ1 mol N2(g)��3 mol H2(g)����һ�ܱ������У��ڴ�������ʱ���з�Ӧ����÷�Ӧ�ų��������ߣߣߣߣ�92 kJ(����ڡ������ڡ���С�ڡ�)��ԭ���ǣߣߣߣߣߣߣߣ��������������H������(������С�����䡱)��
(2)��֪���ֱ��ƻ�1 mol N��N����1 mol H��H����Ҫ���յ�����Ϊ��946 kJ��436 kJ�����ƻ�1 mol N��H����Ҫ���յ�����Ϊ�ߣߣߣߣߣ�kJ��
(3)N2H4����Ϊ��NH3�����е�H����NH2ȡ���IJ������������N2H4(g)Ϊȼ�ϣ�NO2Ϊ����������N2��H2O(g)��
��֪��N2(g)��2O2(g)===2NO2(g) ��H1����67��7 kJ��mol��1
N2H4(g)��O2(g)===N2(g)��2H2O(g) ��H2����534 kJ��mol��1��
��1 mol N2H4��ȫ��Ӧ���Ȼ�ѧ����ʽΪ ��
����ijǦ���ص�����������DZ�ĥ��������ͼװ�����ʵ�飬ʶ�����Ǧ���ص���������
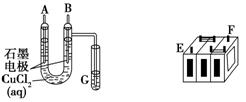
(1)��A��E��B��F����B�缫���� ����ӦʽΪ ����˵��FΪ������
��2����Ǧ���ع���ʱ���ŵ磩����E���ڵ缫�ĵ缫��ӦʽΪ�� �����ʱ�ü�����ӵ�Դ�� ��������
��3�����øõ�ص��Cu(NO3)2 ��Һ�����ⷽ��ʽΪ
����0.2mol���ӷ���ת�ƣ����������ĵ�PbO2�����ʵ����� ��Ҫ��CuSO4��Һ�ָ�ԭ���������������� ,����Ϊ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com