
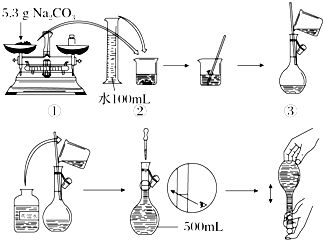
 ��Na2CO3?10H2O���壬����0.2mol?L-1��Na2CO3��Һ480mL��
��Na2CO3?10H2O���壬����0.2mol?L-1��Na2CO3��Һ480mL��| n |
| V |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 773Kʱ���������ʵ�����H2O��CO����1L���ܱ������У�������Ӧ��H2O��g��+CO��g��?H2��g��+CO2��g����H��0����CO��ת������ͼ��ʾ������˵������ȷ���ǣ�������
773Kʱ���������ʵ�����H2O��CO����1L���ܱ������У�������Ӧ��H2O��g��+CO��g��?H2��g��+CO2��g����H��0����CO��ת������ͼ��ʾ������˵������ȷ���ǣ�������| A��t1ʱCO2��Ũ�ȴ���t2ʱCO2��Ũ�� |
| B��773Kʱ���÷�Ӧ�Ļ�ѧƽ�ⳣ��Ϊ0.75 |
| C��773Kʱ��������ƽ����ϵ���ٳ���CO����ѧƽ�ⳣ����С |
| D��������ƽ����ϵ���ȣ���ѧƽ�ⳣ����С |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��֪��һ��̼ԭ�����������ǻ�ʱ�Զ���ˮ�γ�ȩ��������
��֪��һ��̼ԭ�����������ǻ�ʱ�Զ���ˮ�γ�ȩ�������� ����A��F�����л��������ͼ��ʾ��ת����ϵ���ݴ�����������⣺
����A��F�����л��������ͼ��ʾ��ת����ϵ���ݴ�����������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��m��
| ||
B��m��
| ||
C��m��
| ||
D��m=
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com