
����Ŀ��1830�꣬��仯ѧ������˹��ķ��һ������ʯ�еõ���һ����Ԫ�أ����Է��ȵ�˿Ů��![]() ��֮������Ϊ������������
��֮������Ϊ������������![]() ������;�ܹ㣬��ұ����ҵ������һ��������������
������;�ܹ㣬��ұ����ҵ������һ��������������
��1����̬��ԭ��������ߵĵ���ռ�ݵ��ܼ�����Ϊ_________��
��2�����ݼ۵��ӻ��������Ʋ�![]() �����幹����______________��
�����幹����______________��
��3���ٻ�����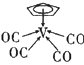 �У�����ԭ��V�ļ۵����������ṩ�ĵ�����֮��Ϊ18������
�У�����ԭ��V�ļ۵����������ṩ�ĵ�����֮��Ϊ18������![]() ������ɣ������ṩ���Ǵ�м��ĵ��ӣ���������
������ɣ������ṩ���Ǵ�м��ĵ��ӣ���������![]() ���еĴ�м��ɱ�ʾΪ_______�������籽�д�м��ɱ�ʾΪ
���еĴ�м��ɱ�ʾΪ_______�������籽�д�м��ɱ�ʾΪ![]() ��
��
�ڴӵ縺�ԽǶȽ���![]() ������ʱ����λԭ����C������O��ԭ����___________________��
������ʱ����λԭ����C������O��ԭ����___________________��
��4��2-�ϻ���������������ͼ�ף�����Ч����Ѫ�ǵ�����ҩ�����Ҫ�ɷ֡�
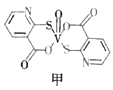
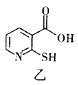
��2-�ϻ����������������![]() ����_________������������������������С������V��O�������������е�ԭ�ӵ��ӻ���ʽ��_________________��
����_________������������������������С������V��O�������������е�ԭ�ӵ��ӻ���ʽ��_________________��
����ͬ�����£�2-�ϻ����ᣨ��ͼ�ң���ˮ���ܽ��Ժ���2-�ϻ���������������ԭ����____��
��5��ij����������ľ�����ͼ��
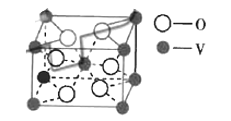
��������λ��Ϊ_____________��
����֪��������Ϊ������������������ķ�ԭ�Ӻ���ԭ�Ӽ�ĺ˼��Ϊ![]() �������ܶ�Ϊ
�������ܶ�Ϊ![]() �����е����ϵ�������ԭ�ӵĺ˼��Ϊ__________cm.
�����е����ϵ�������ԭ�ӵĺ˼��Ϊ__________cm.
���𰸡�3d ֱ���� ![]() O�ĵ縺�Դ���C���Ե��ӵ���������ǿ С�� sp2 �γɷ��Ӽ���� 3
O�ĵ縺�Դ���C���Ե��ӵ���������ǿ С�� sp2 �γɷ��Ӽ���� 3 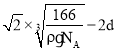
��������
����23��ԭ�ӣ���������Ų�ʽΪ��![]() �����ݹ�ʽ����
�����ݹ�ʽ����![]() ���ӻ���ʽΪ��
���ӻ���ʽΪ��![]() ��Ϊsp�ӻ���������
��Ϊsp�ӻ��������� �У�����ԭ��V�ļ۵����������ṩ�ĵ�����֮��Ϊ18�����ڷ��ļ۵�������5����ÿ��CO��C�����ṩ2�����ӣ�������
�У�����ԭ��V�ļ۵����������ṩ�ĵ�����֮��Ϊ18�����ڷ��ļ۵�������5����ÿ��CO��C�����ṩ2�����ӣ�������![]() �ṩ
�ṩ![]() �����ӣ��ָ���������5��ԭ�ӹ��ɵģ� O�ĵ縺�Դ���C���Ե��ӵ�����������ǿ��������
�����ӣ��ָ���������5��ԭ�ӹ��ɵģ� O�ĵ縺�Դ���C���Ե��ӵ�����������ǿ��������![]() ������ȡͷ��ͷ�ķ�ʽ����������˫����
������ȡͷ��ͷ�ķ�ʽ����������˫����![]() ������ȡ�粢��ķ�ʽ�������̣����ݷ���������ľ�����֪������ֱ�������ķ�ԭ����3�������ݽṹ��֪������߳���a����
������ȡ�粢��ķ�ʽ�������̣����ݷ���������ľ�����֪������ֱ�������ķ�ԭ����3�������ݽṹ��֪������߳���a����![]() ��=2d+
��=2d+![]() �����������ϵ�������ԭ�ӵĺ˼�ࣩ��
�����������ϵ�������ԭ�ӵĺ˼�ࣩ��
��1������23��ԭ�ӣ���������Ų�ʽΪ��![]() ����������Ų���ѭ�������ԭ��������4s������3d��������ܼ���3d��
����������Ų���ѭ�������ԭ��������4s������3d��������ܼ���3d��
�ʴ�Ϊ��3d��
��2�����ݹ�ʽ����![]() ���ӻ���ʽΪ��
���ӻ���ʽΪ��![]() ��Ϊsp�ӻ����Ʋ�
��Ϊsp�ӻ����Ʋ�![]() �����幹����ֱ���ͣ�
�����幹����ֱ���ͣ�
�ʴ�Ϊ��ֱ���ͣ�
��3���������� �У�����ԭ��V�ļ۵����������ṩ�ĵ�����֮��Ϊ18�����ڷ��ļ۵�������5����ÿ��CO��C�����ṩ2�����ӣ�������
�У�����ԭ��V�ļ۵����������ṩ�ĵ�����֮��Ϊ18�����ڷ��ļ۵�������5����ÿ��CO��C�����ṩ2�����ӣ�������![]() �ṩ
�ṩ![]() �����ӣ��ָ���������5��ԭ�ӹ��ɵģ��ʸ�����Ĵ������ɱ�ʾΪ��
�����ӣ��ָ���������5��ԭ�ӹ��ɵģ��ʸ�����Ĵ������ɱ�ʾΪ��![]() ������
������![]() ������ʱ������O�ĵ縺�Դ���C���Ե��ӵ�����������ǿ��������λԭ����C������O��
������ʱ������O�ĵ縺�Դ���C���Ե��ӵ�����������ǿ��������λԭ����C������O��
�ʴ�Ϊ��![]() ��O�ĵ縺�Դ���C���Ե��ӵ���������ǿ��
��O�ĵ縺�Դ���C���Ե��ӵ���������ǿ��
��4��2-�ϻ����������������V��O������![]() ������ȡͷ��ͷ�ķ�ʽ����������
������ȡͷ��ͷ�ķ�ʽ����������![]() ��˫����
��˫����![]() ������ȡ�粢��ķ�ʽ�������̣���
������ȡ�粢��ķ�ʽ�������̣���![]() ����С��V��O�������ٸ������У��γ���̼��˫���������ӻ���ʽ��sp2�ӻ���
����С��V��O�������ٸ������У��γ���̼��˫���������ӻ���ʽ��sp2�ӻ���
�ʴ�Ϊ��С�ڣ�sp2��
��5����������λ�������ݷ���������ľ�����֪������ֱ�������ķ�ԭ����3������������λ����3�����ݽṹ��֪������߳���a����![]() ��=2d+
��=2d+![]() �����������ϵ�������ԭ�ӵĺ˼�ࣩ��
�����������ϵ�������ԭ�ӵĺ˼�ࣩ��![]() ��
��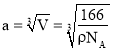 ����
����![]() =����߳���a����
=����߳���a����![]() ��-2d��
��-2d�� ��
��
�ʴ�Ϊ��3��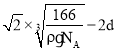 ��
��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��H2S2O3��һ�����ᣬʵ��������0.01mol��L-1��Na2S2O3��Һ�ζ�I2��Һ�������ķ�ӦΪI2+2Na2S2O3=2NaI+Na2S4O6������˵���������ǣ� ��
A.�õζ����ü�����ָʾ��
B.Na2S2O3�Ǹ÷�Ӧ�Ļ�ԭ��
C.�õζ���ѡ����ͼ��ʾװ��
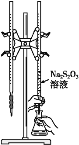
D.�÷�Ӧ��ÿ����1molNaI������ת����Ϊ2NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1���л���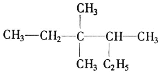 ������Ϊ___________________ ��
������Ϊ___________________ ��
��2�����������dz����ڵ��ƾ��в�ݮ�����ѡ�ӣ�ҡ�����������ζ��ʳ���㾫�����ķ���ʽΪC10H10O2��������ֻ����1���������ұ�����ֻ��һ��ȡ���������ĺ˴Ź�������ͼ����6���źŷ壬�����֮��Ϊ1�U2�U2�U1�U1�U3�����ĺ������ͼ���¡�
��д�����������Ľṹ��ʽ��________________________��
��3��������A��C��Hԭ����֮����4��5��1 mol A����������ȫȼ������8 mol CO2��A������ֻ��7��̼ԭ��һ����ƽ�棬A�Ľṹ��ʽ��____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
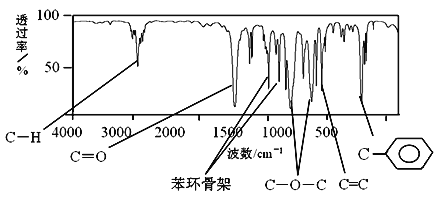
����Ŀ���ȱ���Ⱦ�ϡ�ҽҩ��ҵ���й㷺��Ӧ�ã�ijʵ��С����������װ�úϳ��ȱ���֧���õ�����̨����ʡ�ԣ���ͨ��һ�������ᴿ�ȱ���
��Ӧ��Ͳ������������б����£�
�ܶ�/g��cm��3 | �е�/�� | ˮ���ܽ��� | |
�� | 0.879 | 80.1 | �� |
�ȱ� | 1.11 | 131.7 | ���� |
�밴Ҫ��ش��������⡣
��1��װ��A����c��������______________��װ��E��������__________________��
��2��ʵ��ʱ��ʹa�е�Ũ���Ỻ�����£��ɹ۲쵽����b�ڵ�������________________��д����Ӧ�����ӷ���ʽ______________________________________��
��3��Ϊ֤�������ͱ���������ȡ�������Ǽӳɷ�Ӧ����С����װ��F˵������װ��F����________֮�䣨����ĸ����F��С�Թ���CCl4��������___________________������ʹ�õ��Լ���______________��
��4����֪D�м���5 mL���������ᴿ���ռ����ȱ�3.0 g�����ȱ��IJ���Ϊ_________%��������λ��Ч���֣���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��п��Һ�������һ�����͵绯ѧ����װ�ã���ͼ��ʾ�������ҺΪ�廯пˮ��Һ�����Һ�ڵ���ʴ��͵�ؼ䲻��ѭ��������˵������ȷ����
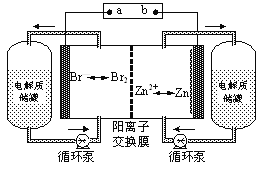
A.���ʱ�缫a���ӵ�Դ�ĸ���
B.�ŵ�ʱ�����ĵ缫��ӦʽΪZn��2e��=Zn2+
C.�ŵ�ʱ������ʴ����е�������Ũ������
D.�����ӽ���Ĥ����ֹBr2��Znֱ�ӷ�����Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
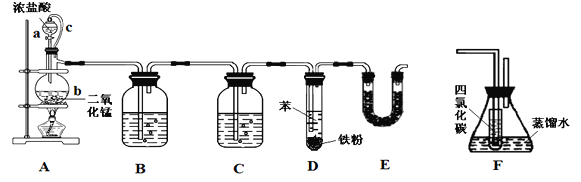
����Ŀ��ʵ������ȼ�շ��ⶨij�ְ�����(CxHyOzNm)�ķ�����ɡ�ȡWg���ְ�������ڴ����г��ȼ�գ����ɶ�����̼��ˮ�͵���������ͼ��ʾװ�ý���ʵ�顣
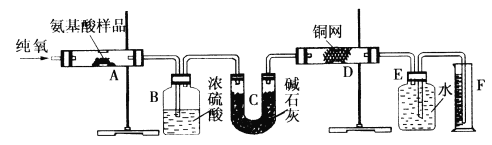
�ش��������⣺
��1��ʵ�鿪ʼʱ������ͨ��һ��ʱ�����������������__________________��
��2������װ������Ҫ���ȵ�������_______ (��д��ĸ)������ʱӦ�ȵ�ȼ_____���ľƾ��ơ�
��3��Aװ���з�����Ӧ�Ļ�ѧ����ʽ��____________________________��
��4��Dװ�õ�������____________________________��
��5����ȡ���������ʱ��Ӧע����_________________����_________________��
��6��ʵ���в�õ��������ΪVmL(��״��)��Ϊȷ���˰�����ķ���ʽ������Ҫ���й�������____________________��
A�����ɶ�����̼���������
B������ˮ������
C��ͨ�����������
D�����������Է�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����t��ʱ��Ag2CrO4(�ٺ�ɫ)��ˮ��Һ�еij����ܽ�ƽ��������ͼ��ʾ����֪AgCl��Ksp��1.8��10��10������˵������ȷ����(����)
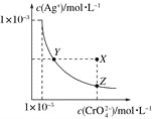
A.t��ʱ��Ag2CrO4��KspΪ1��10��8
B.����Ag2CrO4��Һ�м���K2CrO4����ʹ��Һ��Y���ΪX��
C.t��ʱ��Y���Z��ʱAg2CrO4��Ksp���
D.t��ʱ����0.01 mol��L��1AgNO3��Һ����20 mL 0.01 mol��L��1KCl��0.01 mol��L��1K2CrO4�Ļ����Һ�У�Cl���ȳ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͼ��ʾװ���о�ԭ���ԭ�������������������
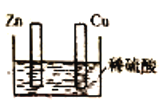
A.Cu����Zn���õ�������ʱ��ͭƬ���������ݳ�
B.Cu����Zn��������ʱ��пƬ���������ݳ�
C.����Cu����Zn���Ƿ��õ������ӣ�װ�������漰���ܷ�Ӧ����ͬ
D.����Cu����Zn���Ƿ��õ������ӣ�װ�ö��ǰѻ�ѧ��ת��Ϊ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1L�����г���0.5mol N2��1.5mol H2������Ӧ��N2��g��+3H2��g��![]() 2NH3��g����H ="-92.4" kJmol-1�����й��ڸ÷�Ӧ��˵����ȷ����
2NH3��g����H ="-92.4" kJmol-1�����й��ڸ÷�Ӧ��˵����ȷ����
A. ��Ӧ�ﵽƽ��ʱ���ų�46.2 kJ������
B. �����������ѹ����0.5L������ߵ�λ����ڻ���Ӱٷ������Ӷ��ӿ�����Ӧ���ʣ������淴Ӧ����
C. �κ�ʱ�̾��У�����N2��=3����H2��=2����NH3��
D. ����������������䣬����ͨ��0.5mol N2��1.5mol H2����N2��ת���ʱ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com