
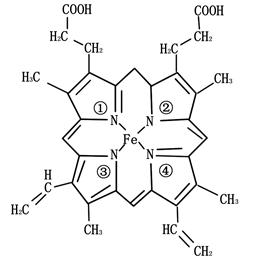
| ¢ŁA”¢B”¢C”¢D”¢E”¢FĪŖ¶ĢÖÜĘŚÖ÷×åŌŖĖŲ£¬Ō×Ó°ė¾¶“󊔹ŲĻµĪŖA£¼D£¼C£¼B£¼F£¼E£» |
| ¢ŚAÓėDŠĪ³ÉµÄ»ÆŗĻĪļ³£ĪĀĻĀĪŖŅŗĢ¬£» |
| ¢ŪBŌŖĖŲŌ×Ó¼Ūµē×Ó£ØĶāĪ§µē×Ó£©ÅŲ¼ĪŖnSnnPn |
| ¢ÜFŌŖĖŲŌ×ÓµÄŗĖĶāpµē×Ó×ÜŹż±Čsµē×Ó×ÜŹż¶ą1£» |
| ¢ŻµŚŅ»µē×ÓÄÜ£ŗF£¼E£» |
| ¢ŽGµÄ»łĢ¬Ō×ÓŗĖĶāÓŠ6øöĪ“³É¶Ōµē×Ó£» |
¢ßHÄÜŠĪ³ÉŗģÉ«£Ø»ņשŗģÉ«£©µÄ ŗĶŗŚÉ«µÄHDĮ½ÖÖ»ÆŗĻĪļ”£ ŗĶŗŚÉ«µÄHDĮ½ÖÖ»ÆŗĻĪļ”£ |
 ÖŠBŌ×Ó²ÉČ”µÄŌӻƹģµĄĄąŠĶĪŖ ”£
ÖŠBŌ×Ó²ÉČ”µÄŌӻƹģµĄĄąŠĶĪŖ ”£ ·Ö×ÓµÄæռ乹ŠĶĪŖ ”£
·Ö×ÓµÄæռ乹ŠĶĪŖ ”£ ¼«Ņ×ČÜÓŚ
¼«Ņ×ČÜÓŚ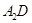 ,ŌŅņŹĒ ”£
,ŌŅņŹĒ ”£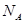 ±ķŹ¾°¢·üŁ¤µĀĀŽ³£Źż£¬¾§°ūµÄ±ß³¤ĪŖa”£¾§°ūµÄøßĪŖh£¬Ōņ¾§°ūµÄĆܶČæɱķŹ¾ĪŖ g/cm3”££ØÓĆÖ»ŗ¬rŗĶ
±ķŹ¾°¢·üŁ¤µĀĀŽ³£Źż£¬¾§°ūµÄ±ß³¤ĪŖa”£¾§°ūµÄøßĪŖh£¬Ōņ¾§°ūµÄĆܶČæɱķŹ¾ĪŖ g/cm3”££ØÓĆÖ»ŗ¬rŗĶ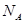 “śŹżŹ½±ķŹ¾£©
“śŹżŹ½±ķŹ¾£©
 £Ø2·Ö£©
£Ø2·Ö£© »ņ
»ņ 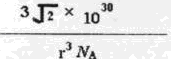
 £»
£»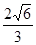 a,1pm=1”Į10-10cm£¬
a,1pm=1”Į10-10cm£¬ a2h=
a2h= =
=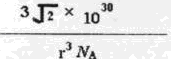


 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®¢Ł¢Ś | B£®¢Ū¢Ü | C£®¢Ł¢Ü | D£®¢Ś¢Ū |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā



²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā

| »ÆѧŹ½ | ¼ü³¤£Ænm | ¼ü½Ē | ·Šµć£Æ”ę |
| H2S | 1.34 | 92.3o | Ņ»60.75 |
| H2Se | 1.47 | 91.0o | Ņ»41.50 |
| ±ąŗÅ | I5£ÆkJ”¤mol-1 | I6£ÆkJ”¤mol-1 | I7£ÆkJ”¤mol-1 | I8£ÆkJ”¤mol-1 |
| A | 6990 | 9220 | 11500 | 18770 |
| B | 6702 | 8745 | 15455 | 17820 |
| C | 5257 | 6641 | 12125 | 13860 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®CS2ĪŖVŠĪµÄ¼«ŠŌ·Ö×Ó | B£®ClO3-µÄæռ乹ŠĶĪŖĘ½ĆęČż½ĒŠĪ |
| C£®SF6ÖŠµÄæռ乹ŠĶĪŖÕżĖÄĆęĢåŠĪ | D£®SiF4ŗĶSO32-µÄÖŠŠÄŌ×Ó¾łĪŖsp3ŌÓ»Æ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com