
A���٢ڢۢܢݡ�����������������B���٢ۢܢ�
C���ۢܢݡ���������������������D���ۢ�
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���� | ����ʽ | ��ɫ��״̬ | �ܽ���(g) | �۵�(��) | �ܶ�(g/cm3) |
�Ҷ��� | H | ��ɫ | 8.6(20��) | 189.5 | 1.900 |
��ˮ���Ҷ��� | H | ��ɫ���� | ���� | 101.5 | 1.650 |
ij��ѧС��ͬѧΪr�о�����(�Ҷ���ĽṹHOOC��COOH)�Ļ�ѧ���ʣ���������ʵ�顣
(1)��С��ͬѧ��ʢ��5 mL�Ҷ��ᱥ����Һ���Թ��е���3�������ữ��0.5��(��������)��KMnO4��Һ�����۲쵽����Ϊ_____________���ɴ˿���֪�Ҷ������___________(������ԡ���ԭ�ԡ�)����������Ԫ�ص���Ҫ���ڼ�̬������___________��
(2)��֪����ֽ�Ļ�ѧ����ʽΪH![]() H2O+CO2��+CO����
H2O+CO2��+CO����
��С��ͬѧΪ����֤�������ȷֽ�IJ���������ͼ��ʾװ�õ�ʵ�顣
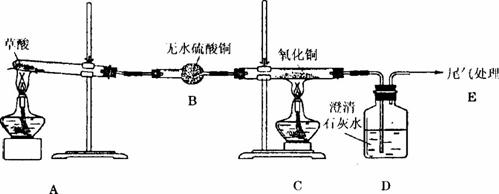
ʵ��ǰ����ͬѧ����ò���Ʒ�����ʵ�飬���ҵķ��ԣ���ԭ����____________________ ____________________����ͬѧ������ʵ�鲻�ܼ��������̼���壬������B��C֮�����4��Dװ�ã����μ�����Լ���___________��___________��_____________��Ũ���ᣬ���е�3��Dװ�õ�������_______________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com