
��14�֣�ij������Ԫ�ص�ԭ������������Ϊ������2�����䵥�ʼɷ������·�Ӧ��
�� + �� �� + �� + ˮ��
�� + �� + ˮ��
��1������ΪNO2��
�ټ����ҷ�Ӧ�Ļ�ѧ����ʽΪ ��
�ڻ������NO2�Ķ�����N2O4�����������£�N2H4����ȼ�ϣ���֪��
N2(g)��2O2(g)��2NO2(g) ��H ����67.7kJ��mol-1
N2H4(g)��O2(g)��N2(g)��2H2O(g) ��H ����534.0kJ��mol-1
2NO2(g) N2O4(g) ��H ����52.7kJ��mol-1
N2O4(g) ��H ����52.7kJ��mol-1
��д����̬�£�N2H4������̬������������ȼ�����ɵ�������̬ˮ���Ȼ�ѧ����ʽ��
___________________________________________________��
�����ֳ��������㷺���ڻ���ƽ������л��ϳɼ�ȼ�ϵ�ء���ȼ�ϵ��ԭ������ͼ��ʾ���ұߵ缫Ϊ �������������������������ߵ缫�Ϸ����ĵ缫��ӦʽΪ ��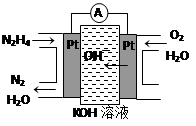
��2������ΪSO2��
�ٰ��ҵ�������ͭ�����У��۲쵽�������� ��
��SO2�����ж��������SO2���峣��������NaOH��Һ���գ�д������Һ������Ũ���ɴ�С��˳�� ��
����������ԭ��Ӧ�Ĺ����У�������Ӧ�ͻ�ԭ��Ӧͬʱ�������йط�Ӧ��
SO2��2e����2H2O = SO42����4H����Ӧ��˵��������� ��
| A���÷�ӦΪ������Ӧ |
| B��������Ӧ����ת�Ƶ������ʵ���Ϊ0.05mol����������Һ��PHֵΪ1 |
| C��Fe2(SO4)3��Ʒ��������Һ����ʹ������Ӧ���� |
| D��ͨ��Cl2�ή��SO2��Ư������ |
 ��һ������ĩ�ٷֳ�̾�ϵ�д�
��һ������ĩ�ٷֳ�̾�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �� |
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��12��)ij������Ԫ�ص�ԭ������������Ϊ������2�����䵥�ʼɷ������·�Ӧ����+����+��+ˮ��
��1������ΪNO2���ټ����ҷ�Ӧ�Ļ�ѧ����ʽΪ ��
��NO2���������Ҫȼ�ϡ��£�N2H4������ȼ������֪��
N2(g)+ 2O2(g)��2NO2(g) ��H= +67.7 kJ��mol-1��
2N2H4(g)+ 2NO2(g)��3N2(g) + 4H2O(g) ��H=��1135.7 kJ��mol-1��
д��ȼ�ϡ��£�N2H4��ȼ�����ɵ�����ˮ�������Ȼ�ѧ����ʽ�� ��
��2������ΪSO2��
�ٰ��ҵ�������ͭ���壬�۲쵽�������� ��
��SO2��ʹ����KMnO4��Һ�Ϻ�ɫ��ȥ������������ӷ���ʽ��
MnO4- +
SO2 +
=
Mn2+ +
SO42- +
H+
��SO2��һ�������£�������2SO2(g)+O2(g)2SO3(g) ��H<0��Ӧ���÷�Ӧ�Ļ�ѧƽ�ⳣ��K= �������ʽ������Ӧ��ƽ��ʱ�����ı�����һ������x�������ͼ�����ߵ��� ������ţ���
a��x��ʾ�¶ȣ�y��ʾSO2�����ʵ���
b��x��ʾѹǿ��y��ʾSO2��ת����
c��x��ʾSO2�����ʵ�����y��ʾO2�����ʵ���
d��x��ʾSO3�����ʵ�����y��ʾ��ѧƽ�ⳣ��K
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ�������������и�����ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��12��)ij������Ԫ�ص�ԭ������������Ϊ������2�����䵥�ʼɷ������·�Ӧ����+�� ��+��+ˮ��
��+��+ˮ��
��1������ΪNO2���ټ����ҷ�Ӧ�Ļ�ѧ����ʽΪ  ��
��
��NO2���������Ҫȼ�ϡ��£�N2H4������ȼ������֪��
N2(g) + 2O2(g)��2NO2(g) ��H= +67.7 kJ��mol-1��
2N2H4(g) + 2NO2(g)��3N2(g) + 4H2O(g) ��H=��1135.7 kJ��mol-1��
д��ȼ�ϡ��£�N2H4��ȼ�����ɵ�����ˮ�������Ȼ�ѧ����ʽ��  ��
��
��2������ΪSO2��
�ٰ��ҵ�������ͭ���壬�۲쵽�������� ��
��SO2��ʹ����KMnO4��Һ�Ϻ�ɫ��ȥ������������ӷ���ʽ�� MnO4- +
MnO4- +  SO2 +
SO2 +  =
=  Mn2+ +
Mn2+ +  SO42- +
SO42- +  H+
H+
��SO2��һ�������£�������2SO2(g)+O2(g) 2SO3(g) ��H< 0��Ӧ���÷�Ӧ�Ļ�ѧƽ�ⳣ��K= �������ʽ������Ӧ��ƽ��ʱ�����ı�����һ������x�������ͼ�����ߵ��� ������ţ���
2SO3(g) ��H< 0��Ӧ���÷�Ӧ�Ļ�ѧƽ�ⳣ��K= �������ʽ������Ӧ��ƽ��ʱ�����ı�����һ������x�������ͼ�����ߵ��� ������ţ���
a��x��ʾ�¶ȣ�y��ʾSO2�����ʵ���
b��x��ʾѹǿ��y��ʾSO2��ת����
c��x��ʾSO2�����ʵ�����y��ʾO2�����ʵ���
d��x��ʾSO3�����ʵ�����y��ʾ��ѧƽ�ⳣ��K
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ������ʡ�������ߴ�ģ�⿼�Ի�ѧ�Ծ��������棩 ���ͣ������
��14�֣�ij������Ԫ�ص�ԭ������������Ϊ������2�����䵥�ʼɷ������·�Ӧ��
�� + �� �� + �� + ˮ��
�� + �� + ˮ��
��1������ΪNO2��
�ټ����ҷ�Ӧ�Ļ�ѧ����ʽΪ ��
�ڻ������NO2�Ķ�����N2O4�����������£�N2H4����ȼ�ϣ���֪��
N2(g)��2O2(g)��2NO2(g) ��H ����67.7kJ��mol-1
N2H4(g)��O2(g)��N2(g)��2H2O(g) ��H ����534.0kJ��mol-1
2NO2(g) N2O4(g)
��H ����52.7kJ��mol-1
N2O4(g)
��H ����52.7kJ��mol-1
��д����̬�£�N2H4������̬������������ȼ�����ɵ�������̬ˮ���Ȼ�ѧ����ʽ��
___________________________________________________��
�����ֳ��������㷺���ڻ���ƽ������л��ϳɼ�ȼ�ϵ�ء���ȼ�ϵ��ԭ������ͼ��ʾ���ұߵ缫Ϊ �������������������������ߵ缫�Ϸ����ĵ缫��ӦʽΪ ��

��2������ΪSO2��
�ٰ��ҵ�������ͭ�����У��۲쵽�������� ��
��SO2�����ж��������SO2���峣��������NaOH��Һ���գ�д������Һ������Ũ���ɴ�С��˳�� ��
����������ԭ��Ӧ�Ĺ����У�������Ӧ�ͻ�ԭ��Ӧͬʱ�������йط�Ӧ��
SO2��2e����2H2O = SO42����4H����Ӧ��˵��������� ��
A���÷�ӦΪ������Ӧ
B��������Ӧ����ת�Ƶ������ʵ���Ϊ0.05mol����������Һ��PHֵΪ1
C��Fe2(SO4)3��Ʒ��������Һ����ʹ������Ӧ����
D��ͨ��Cl2�ή��SO2��Ư������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com