

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ��� | ʵ�鷽�� | ������ |
| A | �������� | ������ɫ���壬����������þ����Ԫ�� |
| B | ����NaOH ��Һ | ����ɫ���������������Ԫ�� |
| C | ���������������Һ���ټ�������NaOH��Һ | ������ɫ����������þԪ�� |
| D | ����KSCN ��Һ | ��Һ��Ѫ��ɫ��������Ԫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| ʵ���� | ʵ����� | ʵ������ |
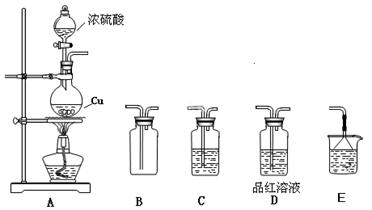
| �� | ȡ������ɫ��ĩX�����Թ�1�У�ע��Ũ���ᣬ�� | ��ɫ��ĩ���ܽ⣬��Һ�ʻ���ɫ�����������ݲ��� |
| �� | ��ȡ������ɫ��ĩX�����Թ�2�У�ע����������ͭ��Һ�������� | �м�������ɫ�������������н϶��ɫ����δ�ܽ� |

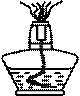

 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
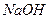 ��Һ���ⶨδ֪Ũ�ȵ������Ũ�ȣ�
��Һ���ⶨδ֪Ũ�ȵ������Ũ�ȣ�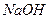 ��Һ����IJ�������Ϊ ��
��Һ����IJ�������Ϊ ��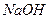 ��Һ�ζ����յ㡣
��Һ�ζ����յ㡣| ʵ���� | ����������mL�� | ��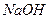 ��Һ�������mL�� ��Һ�������mL�� |
| 1 | 20.00 | 18.20 |
| 2 | 17.10 | |
| 3 | 16.90 |
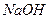 ��Һǰδ��ϴ
��Һǰδ��ϴ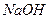 ��Һ�ĵζ��ܼ��첿�������ݣ��ζ��յ����ʱδ��������
��Һ�ĵζ��ܼ��첿�������ݣ��ζ��յ����ʱδ��������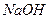 ��Һ�ĵζ��ܼ��첿��û�����ݣ��ڵζ��յ����ʱ���ּ��첿��������
��Һ�ĵζ��ܼ��첿��û�����ݣ��ڵζ��յ����ʱ���ּ��첿���������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
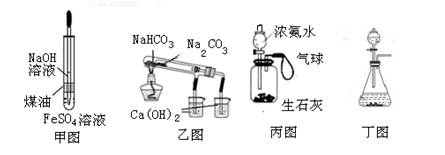
| A���ü�ͼװ���Ʊ����������������� |
| B������ͼװ����֤NaHCO3��Na2CO3�����ȶ��� |
| C���ñ�ͼװ�ÿ���ʵ��ʹ�������� |
| D���ö�ͼװ�ÿ�������ʵ������ȡ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com