

| ||



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| A”¢Fe£ØOH£©3½ŗĢåÓŠ½ĻĒæµÄĪüø½×÷ÓĆ£¬ŹĒŅņĪŖ½ŗĮ£ÓŠ½Ļ“óµÄ±ķĆ껿 |
| B”¢°×Į×ČŪ»Æ”¢øɱłĘū»Æ”¢SiO2ČŪ»Æ”¢AlCl3Éż»Ŗ¶¼ŹĒæĖ·ž·Ö×Ó¼ä×÷ÓĆĮ¦ |
| C”¢Ņŗ¾§ŹĒŅ»ÖÖ½éÓŚ¾§ĢåŗĶŅŗĢåÖ®¼äµÄÖŠ¼äĢ¬ĪļÖŹ£¬·Ö×Ó½ĻŠ”£¬ŌŚŠÅĻ¢¼¼ŹõÖŠ×÷ÓĆ½Ļ“ó |
| D”¢µē½āÖŹČŪČŚŹ±Ņ»¶ØÄܵ¼µē£¬ŅņĪŖĘĘ»µĮĖ»Æѧ¼ü²śÉś×ŌÓÉŅĘ¶ÆµÄĄė×Ó |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| ²Ł×÷²½Öč | ¢Ł¼ÓČė1ml | ¢ŚµĪĮ½µĪøĪŌąŃŠÄ„Ņŗ£¬²¢»ģŗĻ¾łŌČ | ¢Ū¼ÓČė2ml3%µÄH2O2£¬»ģŗĻ¾łŌČ | ŹµŃé½į¹ū |
| 1ŗÅŹŌ¹Ü | Ė® | |||
| 2ŗÅŹŌ¹Ü | 5%NaOHČÜŅŗ | |||
| 3ŗÅŹŌ¹Ü | 5%HClČÜŅŗ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 ŅŃÖŖA”¢B”¢C”¢D¶¼ŹĒÖÜĘŚ±ķÖŠµÄ¶ĢÖÜĘŚŌŖĖŲ£¬ĖüĆĒµÄŗĖµēŗÉŹżŅĄ“ĪŌö“ó£®AŌ×Ó”¢CŌ×ÓµÄLµē×Ó²ćÖŠ£¬¶¼ÓŠĮ½øöĪ“³É¶ŌµÄµē×Ó£¬C”¢DĶ¬Ö÷×壮E”¢F¶¼ŹĒµŚĖÄÖÜĘŚŌŖĖŲ£¬EŌ×ÓŗĖĶāÓŠ4øöĪ“³É¶Ōµē×Ó£¬FŌ×ÓµÄĶāĪ§µē×ÓÅŲ¼Ź½ĪŖ3d104s1£®ĒėĢīæÕ£ŗ
ŅŃÖŖA”¢B”¢C”¢D¶¼ŹĒÖÜĘŚ±ķÖŠµÄ¶ĢÖÜĘŚŌŖĖŲ£¬ĖüĆĒµÄŗĖµēŗÉŹżŅĄ“ĪŌö“ó£®AŌ×Ó”¢CŌ×ÓµÄLµē×Ó²ćÖŠ£¬¶¼ÓŠĮ½øöĪ“³É¶ŌµÄµē×Ó£¬C”¢DĶ¬Ö÷×壮E”¢F¶¼ŹĒµŚĖÄÖÜĘŚŌŖĖŲ£¬EŌ×ÓŗĖĶāÓŠ4øöĪ“³É¶Ōµē×Ó£¬FŌ×ÓµÄĶāĪ§µē×ÓÅŲ¼Ź½ĪŖ3d104s1£®ĒėĢīæÕ£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| A”¢1 molCO2ĪŖ22.4L |
| B”¢±ź×¼×“æöĻĀ£¬1molĖ®Ģå»żĪŖ22.4L |
| C”¢ĻąĶ¬×“æöĻĀ£¬1molH2ŗĶO2ĖłÕ¼Ģå»żĻąĶ¬ |
| D”¢Ö»ÓŠŌŚ±ź×¼×“æöĻĀĘųĢåĦ¶ūĢå»ż²ÅŹĒ22.4mol/L |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 ijĶ¬Ń§Éč¼ĘĮĖČēĶ¼ĖłŹ¾µÄŹµŃé×°ÖĆĄ““ÖĀŌµŲ²ā¶ØµēŹÆÖŠĢ¼»ÆøʵÄÖŹĮæ·ÖŹż£®
ijĶ¬Ń§Éč¼ĘĮĖČēĶ¼ĖłŹ¾µÄŹµŃé×°ÖĆĄ““ÖĀŌµŲ²ā¶ØµēŹÆÖŠĢ¼»ÆøʵÄÖŹĮæ·ÖŹż£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 ĄūÓĆČēĶ¼ĖłŹ¾×°ÖĆŹÕ¼ÆŅŌĻĀ8ÖÖĘųĢå£ØĶ¼ÖŠÉÕĘæµÄĪ»ÖĆ²»µĆ±ä»Æ£©
ĄūÓĆČēĶ¼ĖłŹ¾×°ÖĆŹÕ¼ÆŅŌĻĀ8ÖÖĘųĢå£ØĶ¼ÖŠÉÕĘæµÄĪ»ÖĆ²»µĆ±ä»Æ£©²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| H2SO4 |
| NH3£®H2O |
| ”÷ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
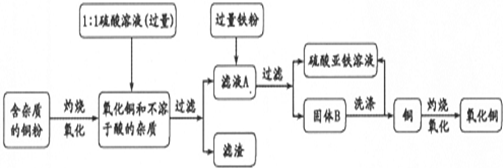
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com