
�״���21����Ӧ����㷺�����ȼ��֮һ��ͨ�����·�Ӧ�����Ʊ��״���
CO(g)+2H2(g)=CH3OH(l) ��H=?
��1����֪��2H2(g)+O2(g)=2H2O(l) ��H=��571.6kJ��mol-1
2CO(g)+O2(g)=2C O2(g) ��H=��566.0kJ��mol-1
O2(g) ��H=��566.0kJ��mol-1
2CH3OH(l)+3O2(g)=2CO2(g)+4H2O(l) ��H=��1453.0kJ��mol-1
���Ʊ��״���Ӧ�ġ�H = kJ��mol-1
��2����װ��Ϊ�ݻ��̶����ܱ���������ͬʱ��θ����ʵ�Ũ�����±���
c(CO) /mol��L-1 | c(H2) /mol��L-1 | c(CH3OH) /mol��L-1 | |
0min | 0.8 | 1.6 | 0 |
2min | 0.6 | y | 0.2 |
4min | 0.3 | 0.6 | 0.5 |
6min | 0.3 | 0.6 | 0.5 |
��Ӧ��2min��4min֮�䣬H2��ƽ����Ӧ����Ϊ________ mol��L��1��min��1��
��Ӧ�ڵ�2minʱ�ı��˷�Ӧ�������ı������������ (����ĸ���)��
A��ʹ�ô��� B�������¶� C������H2��Ũ�� D����СCH3OH(g)��Ũ��
��3�������ݻ��ɱ���ܱ������г���1 mol CO(g)��2 molH2 (g)����CH3OH(g)��H2��ƽ��ת�������¶ȣ�T����ѹǿ��P���ı仯��ͼ��ʾ�����ﵽƽ��״̬A ʱ�����������Ϊ2 L����ʱ�÷�Ӧ��ƽ�ⳣ��Ϊ �����ﵽƽ��״̬B ʱ�������������V(B)= L��

��4��һ���¶��£����ݻ��̶����ܱ������г���һ������H2��CO����t1ʱ�ﵽƽ�⡣t2ʱ���������ݻ�Ѹ������ԭ����2�����������������������£�t3ʱ�ﵽ�µ�ƽ��״̬��֮���ٸı�������������ͼ�в�������t2��t4����Ӧ������ʱ��ı仯���ߡ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2017�챱���и���12���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
һ����������������2mol/L���ᷴӦ��Ϊ�˼ӿ췴Ӧ�����Ҳ�Ӱ���������������������Һ�м���
��3mol/L��������Һ ������CuSO4��s�� �ۼ���һ������ͭ ������CH3COONa��s�� �ݶ���Һ����(�ٶ��������ʲ��ӷ�) ����ӦҺ��ͨ��HCl���� ����������� �ཫ���۸�Ϊ��Ƭ
A���ۢݢ� B���ڢۢ� C���٢ޢ� D���٢ݢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017��ɽ��ʡ�����и���12���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
CO��g��+H2O��g�� H2��g��+CO2��g����H��0���������������������£�����˵����ȷ���ǣ� ��
H2��g��+CO2��g����H��0���������������������£�����˵����ȷ���ǣ� ��
A������������ı��˷�Ӧ��;������Ӧ�ġ�HҲ��֮�ı�
B���ı�ѹǿ��ƽ�ⲻ�����ƶ�����Ӧ�ų�����������
C�������¶ȣ���Ӧ���ʼӿ죬��Ӧ�ų�����������
D������ԭ����н��У���Ӧ�ų�����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�츣��ʡ������ѧ�ڽο�����ѧ�Ծ��������棩 ���ͣ�ѡ����
�μ�������ˮ�����и������ӿ��ܴ������ڵ��� ( )��
A�� Fe3+��Al3+��Cl����NO
Fe3+��Al3+��Cl����NO B��K����Na����I����SO
B��K����Na����I����SO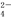
C��Ag+��Ca2+��NH ��NO
��NO D��Na+��Ba2+��CO
D��Na+��Ba2+��CO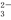 ��SO
��SO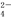
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�츣��ʡ������ѧ�ڽο�����ѧ�Ծ��������棩 ���ͣ�ѡ����
����˵������ȷ���ǣ� ��
A������С�մ�����������ͷ
B�����ܱ������������������ױ���ʴ
C�������ھƾ��ƻ����ϼ����ۻ���������
D������ˮ�еμ���ɫʯ����Һ�ȱ�����ɫ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ���㽭ʡ�����и߶������л�ѧ�Ծ��������棩 ���ͣ�ʵ����
ij��Һ��ֻ���ܺ���K+��NH4+��Fe2+��Al3+��C l����CO
l����CO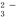 ��SO
��SO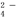 ��NO3-�е����������ӣ�����Ũ�Ⱦ�Ϊ0.1 mol��L��1��ijͬѧ��������ʵ�飺����˵����ȷ���ǣ� ��
��NO3-�е����������ӣ�����Ũ�Ⱦ�Ϊ0.1 mol��L��1��ijͬѧ��������ʵ�飺����˵����ȷ���ǣ� ��
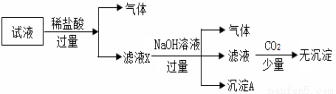
A��ԭ��Һ�п��ܺ���CO ��K+��NH4+
��K+��NH4+
B����ȷ������A�ijɷ�
C����ȷ��ԭ��Һ�� ��Al3+��K +��Cl��
��Al3+��K +��Cl��
D��ԭ��Һ��һ�����ڵ�����ΪNH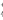 ��Fe2+��SO
��Fe2+��SO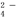 ��NO3-
��NO3-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ���㽭ʡ�����и߶������л�ѧ�Ծ��������棩 ���ͣ�ѡ����
���л�ѧ����ʽ�У���ȷ���ǣ� ��
A�������ȼ���ȡ�H =��890.3 kJ��mol-1�������ȼ�յ��Ȼ�ѧ����ʽ�ɱ�ʾΪ��
CH4(g)+2O2(g)=CO2(g)+2H2O(g) ��H =��890.3 kJ��mol-1
B��һ�������£���0.5molN2��1.5molH2�����ܱ������г�ַ�Ӧ����NH3����akJ�����Ȼ�ѧ����ʽΪ��N2(g)+3H2(g) 2NH3(g) ��H =��2a kJ��mol-1
2NH3(g) ��H =��2a kJ��mol-1
C����101kPaʱ��2gH2��ȫȼ������Һ̬ˮ���ų�285.8kJ��������ˮ�ֽ���Ȼ�ѧ����ʽ��ʾΪ��2H2O(l) =2H2(g)+O2(g) ��H =+571.6 kJ��mol-1
D��HCl��NaOH��Ӧ�к��ȡ�H =��57.3 kJ��mol-1����CH3COOH��NaOH��Ӧ����1molˮʱ�ų�������Ϊ57.3kJ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ���㽭ʡ��Ϫ�и߶���ѧ�����л�ѧ�Ծ��������棩 ���ͣ�ѡ����
����ƽ����ϵ�У����»��ѹ����ʹƽ��������Ӧ�����ƶ����� ( )
A��N2(g)��3H2(g)  2NH3(g) ��H<0
2NH3(g) ��H<0
B��N2(g)��O2(g) 2NO(g) ��H<0
2NO(g) ��H<0
C��C(s)��2H2O(g) CO2(g)��2H2(g) ��H>0
CO2(g)��2H2(g) ��H>0
D��2SO2(g)��O2(g) 2SO3(g) ��H<0
2SO3(g) ��H<0
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ���Ĵ�ʡ�߶��ϰ��ڿ��Ի�ѧ���������棩 ���ͣ�ѡ����
�����£��� pH=3 �������� pH=9 �� NaOH ��Һ��ϣ���Ҫ�õ� pH = 7 ����Һ�����ʱ������ NaOH ��Һ�������Ϊ�� ��
A.1: 200 B. 200 : 1 C. 100 : 1 D.1��100
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com