
��6�֣�����һ��ɫ�Ļ�����ˮ��Һ��ֻ���ܺ������������е������֣�K����Al3����Fe3����Mg2����Ba2����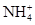 ��Cl��
��Cl��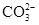 ��
��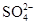 ����ȡ����100 mL��Һ��������ʵ�飺�ٵ�һ�ݼӹ���NaOH��Һ���Ⱥ�ֻ�ռ�������0.02 mol���������ɣ�ͬʱ�õ���Һ�ס����ڼ���Һ��ͨ�����CO2�����ɰ�ɫ���������������ˡ�ϴ�ӡ����գ�����Ϊ1.02 g���۵ڶ��ݼ�����BaCl2��Һ�ð�ɫ��������������������ϴ�ӡ����������Ϊ11.65 g����������ʵ��ش�
����ȡ����100 mL��Һ��������ʵ�飺�ٵ�һ�ݼӹ���NaOH��Һ���Ⱥ�ֻ�ռ�������0.02 mol���������ɣ�ͬʱ�õ���Һ�ס����ڼ���Һ��ͨ�����CO2�����ɰ�ɫ���������������ˡ�ϴ�ӡ����գ�����Ϊ1.02 g���۵ڶ��ݼ�����BaCl2��Һ�ð�ɫ��������������������ϴ�ӡ����������Ϊ11.65 g����������ʵ��ش�
(1)һ�������ڵ�������________________
(2)����ȷ���Ƿ���ڵ�������
(3)��ȷ��K���Ƿ����________(��ǡ���)���жϵ�������
��6��,ÿС��2�֣�
(1)Fe3+ Mg2+ Ba2+ CO32- (2)Cl��
(3) �ǡ���Һ�п϶����ڵ�������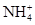 ��Al3����SO42-�������㣬
��Al3����SO42-�������㣬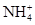 ��Al3�����ʵ�������0.02 mol��SO42-���ʵ�����0.05 mol..���ݵ���غ㣬K��һ������
��Al3�����ʵ�������0.02 mol��SO42-���ʵ�����0.05 mol..���ݵ���غ㣬K��һ������
��������ԭ��Һ��ɫ�����ų�Fe3���Ĵ��ڣ�
�������NaOH��Һ���Ⱥ��ռ���������ض�Ϊ������NH4����OH��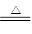 NH3����H2O�������ʵ���Ϊ0.02mol��ͬʱ��������˵��ԭ��Һ�в����ܺ���Mg2+
NH3����H2O�������ʵ���Ϊ0.02mol��ͬʱ��������˵��ԭ��Һ�в����ܺ���Mg2+
����Һ��ͨ�����CO2�����ɰ�ɫ���������֪ԭ��Һ�к���Al3����Al3+4OH��=AlO2����2H2O��AlO2����CO2��2H2O=Al(OH)3��+HCO3����2Al(OH)3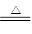 Al2O3��3H2O����Al2O3������Ϊ1.02 g���ɵ�ԭ��Һ��Al3�������ʵ���Ϊ0.02mol������Al3���Ĵ��ڣ�ͬʱ�ɷ���CO32-�Ĵ��ڣ�˫ˮ�ⷴӦ����
Al2O3��3H2O����Al2O3������Ϊ1.02 g���ɵ�ԭ��Һ��Al3�������ʵ���Ϊ0.02mol������Al3���Ĵ��ڣ�ͬʱ�ɷ���CO32-�Ĵ��ڣ�˫ˮ�ⷴӦ����
ԭ��Һ�м�����BaCl2��Һ�ð�ɫ�����Ҳ��������ᣬ��֪ԭ��Һ�к���SO42-(ͬʱҲ���ų���Ba2+���ڵĿ�����)��SO42����Ba2��=BaSO4����������Ϊ11.65 g�������ԭ��Һ�е�SO42-���ʵ�����0.05 mol��
�ۺ�������Ϣ���Ծɲ���ȷ���Ƿ���ڵ�������Cl�������ݵ���غ��֪�ض�����K��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�켪��ʡ������ʮһ�и�����ѧ���ڳ����Ի�ѧ�Ծ����������� ���ͣ������
��6�֣�����һ��ɫ�Ļ�����ˮ��Һ��ֻ���ܺ������������е������֣�K����Al3����Fe3����Mg2����Ba2����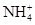 ��Cl��
��Cl��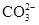 ��
�� ����ȡ����100 mL��Һ��������ʵ�飺�ٵ�һ�ݼӹ���NaOH��Һ���Ⱥ�ֻ�ռ�������0.02 mol���������ɣ�ͬʱ�õ���Һ�ס����ڼ���Һ��ͨ�����CO2�����ɰ�ɫ���������������ˡ�ϴ�ӡ����գ�����Ϊ1.02 g���۵ڶ��ݼ�����BaCl2��Һ�ð�ɫ��������������������ϴ�ӡ����������Ϊ11.65 g����������ʵ��ش�
����ȡ����100 mL��Һ��������ʵ�飺�ٵ�һ�ݼӹ���NaOH��Һ���Ⱥ�ֻ�ռ�������0.02 mol���������ɣ�ͬʱ�õ���Һ�ס����ڼ���Һ��ͨ�����CO2�����ɰ�ɫ���������������ˡ�ϴ�ӡ����գ�����Ϊ1.02 g���۵ڶ��ݼ�����BaCl2��Һ�ð�ɫ��������������������ϴ�ӡ����������Ϊ11.65 g����������ʵ��ش�
(1)һ�������ڵ�������________________
(2)����ȷ���Ƿ���ڵ�������
(3)��ȷ��K���Ƿ����________(��ǡ���)���жϵ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(9��)����һ������ˮ��Һ��ֻ���ܺ������������е������֣�NH4+��K+��Ba2+��CO32-��SO32-��SO42-��Br����I�D����ȡ�ķ�100mL��Һ��������ʵ�飺
�ٵ�һ�ݼ������������ɫ��ζ���壬��������ʹ����ʯ��ˮ����ǣ�
�ڵڶ��ݼ�����BaCl2��Һ�ø������8��24g������������ϴ�ӡ������������Ϊ2��33g��
�۵����ݼӼ����������0��04mol
�ܵ��ķݼ�CCl4�μ�������ˮ����CCl4��δ��ɫ��
�������ʵ�飬�ش���������
�û��Һ�п϶����ڵ������� �������ʵ���Ũ��֮��Ϊ ���϶������ڵ��� ������ȷ������ ��(����������Ӷ���ȷ���������ޡ�)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���㽭ʡ�����и߿���ѧ��ģ�Ծ��������棩 ���ͣ�ѡ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com