
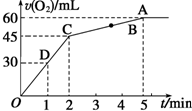
 ����0.1mol MnO2��ĩ��50mL����������Һ����=1.1g•mL-1���У��ڱ�״���·ų�����������ʱ��Ĺ�ϵ��ͼ��ʾ��
����0.1mol MnO2��ĩ��50mL����������Һ����=1.1g•mL-1���У��ڱ�״���·ų�����������ʱ��Ĺ�ϵ��ͼ��ʾ������ ��1����Ӧ����ʽΪ��2H2O2$\frac{\underline{\;MnO_{2}\;}}{��}$2H2O+O2�����÷�ӦΪ�����淴Ӧ����5min���ռ�������������������ӣ�˵������������ȫ�ֽ⣬����ͼ���֪���ɵ������������
��2����Ӧ�ų��������Ϊ���������һ��Ϊ30mL������ͼ���ж���Ҫ��ʱ�䣻
��3����Ӧ�ų��������Ϊ�����������$\frac{3}{4}$ʱ�����ɵ��������Ϊ45mL������ͼ���жϷ�Ӧʱ�䣻
��4������Ũ�ȶԷ�Ӧ���ʵ�Ӱ���ж�A��B��C��D���㷴Ӧ���ʴ�С��
��5�����ŷ�Ӧ�Ľ��У���Һ��Ũ�����ͣ���Ӧ������С��
��6����5min���ռ�������������������ӣ�˵������������ȫ�ֽ⣬����ͼ���֪���ɵ���������������ݷ���ʽ�����������Ũ�ȣ�
��7������m=��V����ԭ��Һ���������ݷ���ʽ����ֽ�Ĺ������⣬��ʱ��Һ����=ԭ��Һ����-�������������������ʱ�������������������
��� �⣺��1���ɷ�Ӧ����ʽΪ��2H2O2$\frac{\underline{\;MnO_{2}\;}}{��}$2H2O+O2�����÷�ӦΪ�����淴Ӧ����5min���ռ�������������������ӣ�˵������������ȫ�ֽ⣬��ͼ���֪���������������Ϊ60mL��
�ʴ�Ϊ��60ml��
��2����ͼ���֪����ʱ����е�1minʱ���������������Ϊ30mL����ʱ���ɵ�����Ϊ�������һ�룬��Ҫ��ʱ��Ϊ1min��
�ʴ�Ϊ��1min��
��3����ͼ���֪�����ɵ������Ϊ60mL����Ӧ�ų��������Ϊ�����������$\frac{3}{4}$ʱ�����ɵ��������Ϊ45mL����Ҫ��ʱ��Ϊ2min��
�ʴ�Ϊ��2min��
��4����Ӧ��Ũ�ȴ�С������Ӧ���ʴ�С�����ŷ�Ӧ�Ľ��У�˫��ˮ��Ũ����С����Ӧ����Ҳ���ż�С��
�ʴ�Ϊ��D��C��B��A��
��5��Ũ��Խ��Ӧ����Խ��֮ԽС�����ŷ�Ӧ���У���Ӧ���Ũ����С����������С��
�ʴ�Ϊ�����ŷ�Ӧ�Ľ��У�˫��ˮ��Ũ����С����Ӧ����Ҳ���ż�С��
��6���ɷ�Ӧ����ʽΪ��2H2O2$\frac{\underline{\;MnO_{2}\;}}{��}$2H2O+O2�����÷�ӦΪ�����淴Ӧ����5min���ռ�������������������ӣ�˵������������ȫ�ֽ⣬
��ͼ���֪���������������Ϊ60mL��
2H2O2$\frac{\underline{\;MnO_{2}\;}}{��}$2H2O+O2����
2mol 22.4L
n��H2O2�� 0.06L
n��H2O2��=$\frac{2mol��0.06L}{22.4L}$=0.00536mol������c��H2O2��=$\frac{0.00536mol}{0.05L}$=0.107 mol•L-1��
�ʴ�Ϊ��0.107 mol•L-1��
��7��ԭ��Һ����Ϊ50mL��1.1g•mL-1=55g��2minʱ�����������ʵ���Ϊ$\frac{0.045L}{22.4L/mol}$=0.002����2H2O2$\frac{\underline{\;MnO_{2}\;}}{��}$2H2O+O2����
��֪�ֽ��H2O2�����ʵ���Ϊ0.002mol��2=0.004mol��ʣ��Ĺ�������Ϊ��0.00536mol-0.004mol����34g/mol=0.04624g����ʱ��Һ����=55g-0.002mol��32g/mol=54.936g���ʴ�ʱ�����������������=$\frac{0.04624g}{54.936g}$��100%=0.084%��
�ʴ�Ϊ��0.084%��
���� ���⿼���������ֽ���������ߣ���Ŀ�ѶȲ�����Ҫ����Ũ�ȶԷ�Ӧ���ʵ�Ӱ�죬��ȷ����ͼ������߱仯�ǽ����Ĺؼ���


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Ba��OH��2��NaHSO4 | B�� | Ba��OH��2��H2SO4 | ||
| C�� | ʯ��ˮ�Ͷ�����̼ | D�� | Ca��HCO3��2��NaOH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
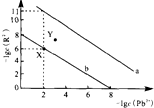 25��ʱ��PbR��R2-ΪSO42-��CO32-���ij����ܽ�ƽ��������ͼ����֪Ksp��PbCO3����Ksp��PbSO4��������˵������ȷ���ǣ�������
25��ʱ��PbR��R2-ΪSO42-��CO32-���ij����ܽ�ƽ��������ͼ����֪Ksp��PbCO3����Ksp��PbSO4��������˵������ȷ���ǣ�������| A�� | ����a��ʾPbCO3 | |
| B�� | ��PbSO4��Na2CO3�ͽ�̿Ϊԭ�Ͽ��Ʊ�Pb | |
| C�� | ��PbSO4��PbCO3��������ʱ����Һ��$\frac{c��S{O}_{4}^{2-}��}{c��C{O}_{3}^{2-}��}$=105 | |
| D�� | ��X���Ӧ�ı�����Һ�м�������Pb��NO3��2����ת��ΪY���Ӧ����Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��-��ѭ���ֽ�ˮ������Ҫ�漰���з�Ӧ��
��-��ѭ���ֽ�ˮ������Ҫ�漰���з�Ӧ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
| ʵ����� | �¶� | 0min | 10min | 20min | 30min | 40min | 50min | 60min |
| 1 | 820�� | 1.0 | 0.80 | 0.67 | 0.57 | 0.50 | 0.50 | 0.50 |
| 2 | 820�� | c1 | 0.60 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| 3 | 800�� | c2 | 0.92 | 0.75 | 0.63 | 0.60 | 0.60 | 0.60 |
| 4 | 800�� | 1.0 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ���� | Fe��OH��2 | Cu��OH��2 | Fe��OH��3 |
| Ksp/25�� | 8.0��10-16 | 2.2��10-20 | 4.0��10-38 |
| ��ȫ����ʱ��pH��Χ | ��9.6 | ��6.4 | 3��4 |
| A�� | ��û����Һ�м����������ۼ��ܹ۲쵽��ɫ�������� | |
| B�� | ��û����Һ����μ���NaOH��Һ�����ȿ�����ɫ���� | |
| C�� | �û����Һ��c��SO42-����{c��Cu2+��+c��Fe2+��+c��Fe3+��}��5��4 | |
| D�� | ��û����Һ�м���������ˮ��������pHΪ3��4��Ȼ����ˣ��ɵõ�������CuSO4��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com