



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
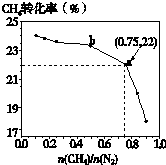 ��֪��3CH4��g��+2N2��g��
��֪��3CH4��g��+2N2��g��| 700�� |
| ���� |
| n(CH4) |
| n(N2) |
A��
| ||
B��
| ||
| C��b���Ӧ��ƽ�ⳣ����a��Ĵ� | ||
| D��a���Ӧ��NH3���������ԼΪ26% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
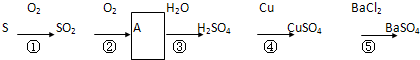
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��ѧʽ | CH3COOH | H2CO3 | HClO | |
| ����ƽ�ⳣ�� | Ka=1.8��10-5 | Ka1=4.3��10-7 | Ka2=5.6��10-11 | Ka=3.0��10-8 |
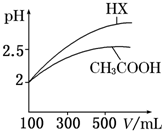 �ش��������⣺
�ش��������⣺| c(H+) |
| c(CH3COOH) |
| c(OH-) |
| c(H+) |
| c(H+)?c(CH3COO-) |
| c(CH3COOH) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��XH4�ķе��YH3�� |
| B��X��W�γɵĻ������Z��W�γɵĻ�����Ļ�ѧ��������ͬ |
| C��Ԫ��Y��W�γɵĻ���������ˮ�����Ư���� |
| D��X��Y�γɵĻ�������������Ӿ��� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com