
����Ŀ��ʵ�����Ե�ʯ��Ҫ�ɷ���CaC2)Ϊԭ���Ʊ���Ȳ��װ����ͼ��ʾ��
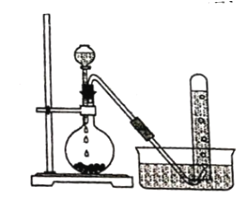
(1)ʵ������ȡ��Ȳ�Ļ�ѧ����ʽΪ ______________��
(2)Ϊ��ȥ��Ȳ�л��е�������������,�ɽ�����ͨ�� ____________ (ѡ����)��
a.����KMnO4��Һ b.CCl4 c.NaOH��Һ
(3)��Ȳ������Ȳ��������ȼ�յĸ��»��棬������______________________ ��
(4)��Ȳ��һ�����������۵õ�CH2=CHC��CCH=CH2��д����������������ͬ��ѧʽ�Ҳ���ʹ���CCl4��Һ��ɫ�����Ը��������Һ��ɫ�����ʵĽṹ��ʽ_________
(5)��Ȳ�ۺϵõ�����Ȳ,����Ȳ__________����(ѡ������������������)��
���𰸡�![]() c ��������
c �������� ![]() ��
��
��������
(1)��ʯ��ˮ��Ӧ������Ȳ���������ƣ�
(2)��ϩ�����ⶼ�ܱ����������Һ���������Ȼ�̼������Ȳ���������⣻�������ƺ����ⷴӦ�������ƺ�ˮ��
(3) ��Ȳ����¶ȸߣ��������������
(4)����ʹ���CCl4��Һ��ɫ�����Ը��������Һ��ɫ��˵���������͵�̼̼˫���������Ȳ����ͼ���
��5��̼̼������̼̼˫��������ֵ��л����ܵ��磻
(1)��ʯ��ˮ��Ӧ������Ȳ���������ƣ���Ӧ����ʽ��![]() ��
��
(2)a.����KMnO4��Һ���������⣬Ҳ��������Ȳ����a����
b.���Ȼ�̼���������⣬��b����
c.�������ƺ����ⷴӦ�������ƺ�ˮ���������ƺ���Ȳ����Ӧ����c��ȷ��
(3) ��Ȳ����¶ȸߣ��������������
(4) CH2=CH-C��C-CH=CH2�ķ���ʽΪC6H6�������Ͷ�Ϊ4���������ӳɷ�Ӧ��˵�������е��͵�˫�������������Խṹ��ʽΪ![]() ��
��
��5����Ȳ���ɾ���Ȳ��nCH![]() CH
CH![]()
![]() ������Ȳ��̼̼������̼̼˫��������֣������ܵ��硣
������Ȳ��̼̼������̼̼˫��������֣������ܵ��硣



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������һ�ֳ��������ĺϳ�·�ߡ�
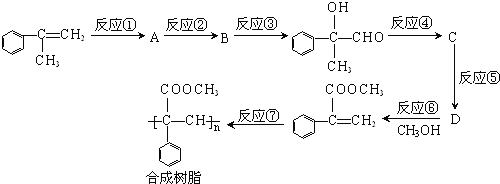
��1���жϸúϳ���֬��__(����������������������)����̼̼˫����
��2����Ӧ�ٵķ�Ӧ������__����Ӧ�ܵķ�Ӧ������__��
��3��д��B��D�Ľṹ��ʽ��B___��D__��
��4��������C������ȥ2����ˮ���γ�һ�ֻ�״�����д���÷�Ӧ�Ļ�ѧ����ʽ��___��
��5����������������D��ͬ���칹����__�֡�
�ٱ����Ϲ�������ȡ����������һ��Ϊ�ǻ������ܷ���������Ӧ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£���20mL0.1molL-1NH4HSO4��Һ����μ���0.1molL-1��NaOH��Һ����Һ����ˮ�������cˮ��H+��������NaOH��Һ������Ĺ�ϵ��ͼ��ʾ�����з�����ȷ���ǣ� ��
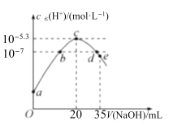
A.c��֮ǰ����Ҫ�ķ�ӦΪNH4++OH-�TNH3H2O
B.b���d���Ӧ��Һ��pH��Ϊ7
C.�����£�Kb��NH3H2O��=5��10-5.4molL-1
D.d����Һ�У�c��Na+��+c��NH4+��=c��SO42-��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ϳɸ߷��ӽ����������ϼ��ķ�Ӧ��
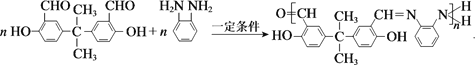 +(2n-1)H2O
+(2n-1)H2O
����˵������ȷ����
A.�ۺ���Ӧ������������ʣ���NaOH�����ڽӴ�
B.�ۺ���ĺϳɷ�ӦΪ���۷�Ӧ
C.1mol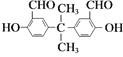 ������������Һ���ÿ�����2mol��
������������Һ���ÿ�����2mol��
D.ͨ�������ⶨ��ƽ����Է����������ɵ���ۺ϶�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʵ��װ�ô�����ǣ� ��
A.��Ȳ����ȡ���ռ�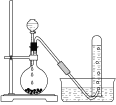 B.����������Ӧ
B.����������Ӧ
C.������������ȡ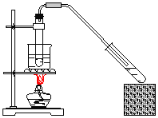 D.��ϩ����ȡ
D.��ϩ����ȡ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������ͬ����Ԫ�أ��䵥�ʺͻ�������������������;�ܹ㡣
SO2�����ڷ���������������Ҳ��һ����Ҫ���䶳���ʡ�ʵ���ҿ�����ͼ��ʾװ���Ʊ�SO2�����ô���SO2�������ʵ�顣
(1)���������Ʊ���SO2���������������СҺ�ζ��ʰ���״����ȥ���������Ʊ�װ�ú����ӳ���װ�ã��뻭������װ�ò�����װ���е��Լ�___________��
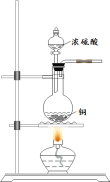

(2)��SO2ͨ��0.1mol/L Ba(NO3)2��Һ�õ���ɫ�������÷�Ӧ�����ӷ���ʽΪ_______��
�ֱ�����к�δ��й�������ˮ����Ba(NO3)2��BaCl2��Һ����������ʵ�飺
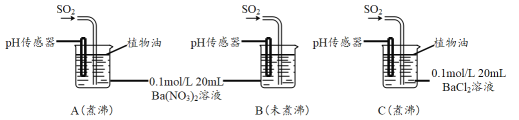
(3)ʵ��C�У�û�й۲쵽��ɫ��������pH��������ʾ��Һ�����ԣ��û�ѧ�����ʾ��ԭ��______________________________��
(4)ʵ��B�г��ְ�ɫ������ʵ��A��ܶࡣ�ɴ˵ó��Ľ�����_________________��
(5)�ⶨˮ���ܽ�O2���������õķ����ǣ�
i����ȡa mLˮ����Ѹ�ټ�������MnSO4��Һ������NaOH��KI��Һ�������������ӣ���ʹ��Ӧ���ȡ�
ii��������Ѹ�ټ������������ᣬ��ʱ��I2���ɡ�
iii����ii������Һ�еμ�2�ε�����ҺΪָʾ������b mol/LNa2S2O3����Һ�ζ����յ㹲������Na2S2O3��ҺV mL��
�йط�Ӧ����ʽΪ��2Mn2+ + O2+ 4OH- = 2MnO(OH)2����Ӧ�ܿ죩
MnO(OH)2 + 2I- + 4H+= Mn2+ + I2 + 3H2O
I2 + 2S2O32- = 2I- + S4O62-
��ˮ���ܽ� O2��������mg/LΪ��λ��Ϊ___________________��
���жϴﵽ�ζ��յ��ʵ������Ϊ __________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��NA���������ӵ�������ֵ������˵����ȷ���ǣ� ��
A.0.1molH2��0.1molI2(g)���ܱ������г�ַ�Ӧ��������ԭ������Ϊ0.2NA
B.���³�ѹ�£�28gFe������Ũ�����ϣ�ת�Ƶ�����Ϊ1.5NA
C.��״����22.4LHF�к��еķ�ԭ����ĿΪNA
D.2gD2O��H2l8O�����������������ΪNA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���к͵ζ��������Բⶨ��������ʵ���Ũ�ȣ������ԲⶨijЩ�����ĺ�����
�ռ�����Ҫ�Ļ���ԭ�ϣ���ҵ�ռ��п��ܺ�������NaCl��Ϊ�ⶨ��ҵ�ռ���Ʒ��NaOH��������������������ʵ�飺
�ٳ�ȡ1. 000g��Ʒ�����Ƴ�250mL����Һ��
����ȡ20.00mL����Һ����0.1000mol/L��������Һ�ζ���
��1����1.000g��Ʒ���250mL����Һ�����õIJ��������У��ձ�������������ͷ�ι� _____________________________________��
��2����ȡ20.00mL����Һ���õ�������_______________________��ѡ�õ�ָʾ��Ϊ_____________________��
��3���ζ����������У��۾�Ӧע��____________________________________________���жϴﵽ�ζ��յ������Ϊ_______________________________________________��
��4����һ��ѧ���ڲⶨʱ��������ι���ͷ������ѧ���ּ���2.80mL����Һ��������ʵ�飬���ֲ���__________(���������)��
��5���ڶ���ѧ���ڵζ������У���С�Ľ���Һ������ƿ���ڱ��ϣ�һѧ������������ˮ��Һ�γ���ȥ������Ϊ��������ʹ���_______ (�ƫ�ߡ�ƫ�ͻ�û��Ӱ��)��
��6��������ѧ�����������εζ������ı����������������������е�2�εζ���ζ��ܵĶ�����ͼ��ʾ������һ����������С�
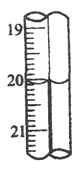
���� | �ζ�ǰ��mL�� | �ζ��� |
1 | 0.40 | 20.10 |
2 | 0.10 | ____________ |
����ѧ������õ��ռ���Ʒ��NaOH����������Ϊ__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij������Ʒ�к��������Ȼ��ƣ������ⶨ��̼���Ƶ������������������ʵ�鷽����
������1����ȡһ�������Ĵ�����Ʒ����֪��ƿ��������Һ������190.720 g����������ͼװ�òⶨ������Ʒ�Ĵ��ȣ�ÿ����ͬʱ����õ�����ƽ�����������

�������� | ������g�� | |
��ƿ+����+���� | ��1�� | 192.955 |
��2�� | 192.764 | |
��3�� | 192.328 | |
��4�� | 192.075 | |
��5�� | 192.075 |
��1�����㴿����Ʒ�Ĵ���ʱ�������������_____________________________����������ݣ�����������6�ζ�����ԭ����________________________________________________��
��2�����㴿����Ʒ�Ĵ���Ϊ_________________________������С������λ����
������2���ⶨ������Ʒ��1.15 g���У�Na2CO3������������һ�ַ�����������������£�
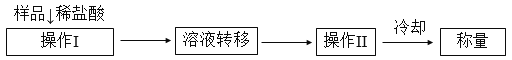
��1����Һת����__________����д�������ƣ�������II��������______________��
��2����ֱ�Ӳⶨ����������____________________��
��3���ⶨ��������Ҫ�������е�����ƽ�������ƾ��ơ�����Ҫ__________��__________���̶����г��������⣩��
��4����ת����Һʱ������Һת�Ʋ���ȫ����Na2CO3���������IJⶨ���__________������ƫС������ƫ����������������
������3��ʵ��װ����ͼ��
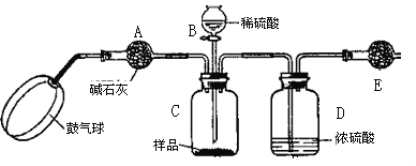
ʵ�鲽�裺
����ͼ����װ�ò���������ҩƷ��
�ڳ�������¼E������m1������ʱע����E�����ˣ���
�۰�����������Լ1���ӡ�
��������E��
�ݴ�Һ©��B�Ļ�������ϡ������ټ���C�кرջ�����
�ް�����������Լ1���ӡ�
�߳�������¼E������m2������ʱע����E�����˼�D�Ҷ˵ij��ڣ���
���ظ�����͢ߵIJ�����ֱ�����θ���ܵ������������䣬��Ϊm3��
����㡣
����պͻش����⣺
��1��C�з�����Ӧ�����ӷ���ʽΪ��______________________________________��B����������Ϊ__________���������Һ©���е����ỻ��Ũ����ͬ�����ᣬ���ԵĽ����__________������ƫ��������ƫ������������������
��2��Ũ�����������_________________����û��D����ʵ����__________������ƫ��������ƫ����������Ӱ��������
��3������ۺ͢�������____________________��____________________����������ٶ��ǿ��ٺã����ǻ������룿Ϊʲô��__________________________________________��
��4��Eװ�õĹ����Լ�Ϊ__________�����ţ�
A����ʯ�� B����ˮ�Ȼ��� C��Ũ���� D����ʯ��
��5��������Ŀ����________________________________________________��
��6�������д�����Ʒ��������������ʽΪ_____________________________________��
��7����ʵ�������������Ҫ�Ľ��ĵط�����ָ���ý�֮����˵��ԭ��
_______________________________________________________________________��
��8��ʵ�黹��������������ʵ�鷽���ⶨ�����д�������������������һ�ֲ�ͬ�Ķ���ʵ�鷽����___________________________________________________________ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com