
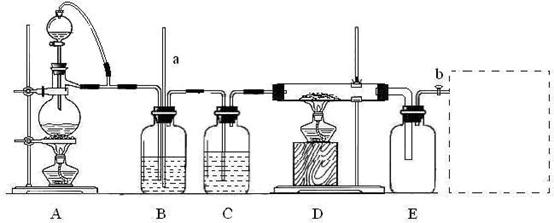
��15�֣�ijУ��ѧ����С��������ͼװ���Ʊ��Ȼ������壬���øù��������Ȼ�����Һ��װ����aΪ���������ܣ�bΪ����������ʵ���пɹ�ѡ�õ��Լ��У��ٶ������̹��壻�����ۣ����Ȼ��ƹ��壻��Ũ�����Ũ���������ˮ����ʯ�ҡ����ϱ������Ȼ���������180��ʱ���������ڳ�ʪ�Ŀ�������ˮ�⡣
�ش��������⣺
��1������װ�������Եķ�����___________________________________��
��2��A�������Ʊ������ӷ���ʽ��_________________________������ɹ�ѡ����Լ�����Ũ���ᣬ��С��ͬѧ���ѹ��Լ�Ҳ����˸�ʵ�飬��Aװ����ѡ�õ��Լ���____(�����)��
��3��ʵ����Bװ�õ�������_________________________________��
��4��D��E�䵼�ܶ��Ҵ֣�ԭ����___________________________��
��5����ʵ���Ƶõ��Ȼ�������������Һ�ķ�����_______________________��
��6������E������߿��в������Ƹ�װ�á���װ����Ӧʢ�ŵ��Լ���______�������пɹ��Լ���ѡ������ţ���

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��㶫ʡ��һ��ѧ�ڻ�ѧ��Ԫ�����Ծ� ���ͣ�ʵ����
��6�֣��ڳ����ʵ�ʵ������У�Ϊ�˽���Һ�е�������ȫȥ����ͨ�����������ij������Լ���ijУ��ѧ����С��Ϊ�˳�ȥ�����к�������Na2SO4��MgCl2�����������ʵ�鲽�裺

�Լ�Ҫ˵�� ��1���������BaCl2����Һ��ԭ��____ ___ ��
��2���������Na2CO3����Һ��ԭ��______ ___ _ _��
��3���μ�ϡ����ֱ��pH=7��Ŀ����____ ___ _ _��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com