
 ���ѾƳ���Na2S2O5������������
���ѾƳ���Na2S2O5���������������� ��1����Na2S2O5��O2�ķ�Ӧ�У����+4����Ϊ+6�ۣ�����0�۽�Ϊ-2�ۣ����ݵ��ӵ�ʧ�غ���м��㣻
��2������ͼ��֪��pH=4.5ʱ����Һ����Ҫ�����������������ʽ���ڣ��ݴ���дˮ�ⷽ��ʽ������ҺpHС��1����Һ����Ҫ���������γɴ��ڣ���������ȶ����ֽ����ɶ���������Ksp[BaSO4]=c��Ba2+��•c��SO42-�����ɼ������Ҫ����c��Ba2+���������������Ũ��c��SO32-�����ݴ˴��⣻
��3�����ݷ�ӦSO2+I2+2H2O=H2SO4+2HI���ɵ�����ʵ����ɼ����������������ʵ�����������Ԫ���غ��ٻ����Na2S2O5���ɴ˼������Ѿ���Ʒ�п��������IJ�������
��� �⣺��1����Na2S2O5��O2�ķ�Ӧ�У����+4����Ϊ+6�ۣ�����0�۽�Ϊ-2�ۣ�1.90g Na2S2O5�����ʵ���Ϊ0.01mol�����ݵ��ӵ�ʧ�غ��֪�ܻ�ԭ���������ʵ���Ϊ0.01mol������״��224mL��
�ʴ�Ϊ��224��
��2������ͼ��֪��pH=4.5ʱ����Һ����Ҫ�����������������ʽ���ڣ�����ˮ�ⷽ��ʽΪNa2S2O5+H2O=2NaHSO3������ҺpHС��1����Һ����Ҫ���������γɴ��ڣ���������ȶ����ֽ����ɶ�������������ᱻ����Ҳ�ᵼ��Ũ��С��
����Ksp[BaSO4]=c��Ba2+��•c��SO42-������֪��Ҫc��Ba2+��=$\frac{Ksp��BaS{O}_{4}��}{c��S{O}_{4}^{2-}��}$=$\frac{1��1{0}^{-10}}{1��1{0}^{-5}}$=10-5mol•L-1������Һ��SO32-�����Ũ��c��SO32-��=$\frac{Ksp��BaS{O}_{3}��}{c��B{a}^{2+}��}$=$\frac{5��1{0}^{-7}}{1{0}^{-5}}$=0.05mol•L-1��
�ʴ�Ϊ��Na2S2O5+H2O=2NaHSO3��������ȶ����ֽ����ɶ������������ᱻ������0.05��
��3�����������֪�����ı�I2��Һ�����Ϊ$\frac{15.98mL+16.02mL}{2}$=16.0mL������I2�����ʵ���Ϊ16.0��10-3L��0.0225mol•L-1=3.6��10-4mol�����ݷ�ӦSO2+I2+2H2O=H2SO4+2HI����֪������������ʵ���Ϊ3.6��10-4mol��SO2������Ϊ64g/mol��3.6��10-4mol=23.04mg���������Ѿ���Ʒ�п��������IJ�����Ϊ$\frac{23.04mg}{0.1L}$=230.4mg•L-1���𣺲�����Ϊ230.4mg•L-1��
���� ���⿼��ͼ����������ʺ����IJⶨ��������ԭ��Ӧ�ζ��ȣ��Ѷ��еȣ���ȷʵ��ԭ���ǽⱾ��ؼ����������ʵ����ʷ������ע��Ԫ�ػ�����֪ʶ�Ļ��ۺ�������ã�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 ��I2����KI��Һ�У������Ƴ�Ũ�Ƚϴ�ĵ�ˮ����Ҫ�Ƿ����˷�Ӧ��I2��aq��+I-��aq��?I3-��aq������ƽ����ϵ�У�I2�����ʵ���Ũ�����¶ȣ�T���Ĺ�ϵ��ͼ��ʾ�������ϵ��κ�һ�㶼����ƽ��״̬��������˵����ȷ���ǣ�������
��I2����KI��Һ�У������Ƴ�Ũ�Ƚϴ�ĵ�ˮ����Ҫ�Ƿ����˷�Ӧ��I2��aq��+I-��aq��?I3-��aq������ƽ����ϵ�У�I2�����ʵ���Ũ�����¶ȣ�T���Ĺ�ϵ��ͼ��ʾ�������ϵ��κ�һ�㶼����ƽ��״̬��������˵����ȷ���ǣ�������| A�� | ����ӦΪ���ȷ�Ӧ | B�� | ƽ�ⳣ����KA��KB | ||
| C�� | ��Ӧ���ʣ�vB��vC | D�� | W��ʱ��v����v�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����ʱ��NiΪ�������Ƽ�Ϊ���� | |
| B�� | ��ƺͻ�ѧ��ԭ����������������ԭ��Ӧ | |
| C�� | ��ѧ������ͨ�磬�ԶƼ��ĵ�����������Ҫ�� | |
| D�� | ��ѧ����H2PO2-��P���ϼ�Ϊ+1����ǿ��ԭ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��

| A�� | 3�ֵ���ͨ�����۷�Ӧ�ۺ� | B�� | �γɸû�����ĵ���ֻ��2�� | ||
| C�� | ����һ�ֵ���Ϊ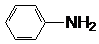 | D�� | ����һ�ֵ���Ϊ1��5-�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������Fe��OH��3��Ӧ��Fe��OH��3+3H+�TFe3++3H2O | |
| B�� | ϡ���������۷�Ӧ��2Fe+6H+�T2Fe3++3H2�� | |
| C�� | ����������Һ��ϡ���ᷴӦ��Ba2++SO42-�TBaSO4�� | |
| D�� | ̼��������ᷴӦ��CO32-+2H+�TH2O+CO2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ͬŨ�ȵ�NaHA��Na2A��Һ�������ϣ�������Һ��pHһ��С��7 | |
| B�� | 0.1 mol•L-1��NaHA��Һ������Ũ��Ϊ��c��Na+������c��HA-����c��H+��c��A2-����c��OH-�� | |
| C�� | ��0.1 mol•L-1��H2A��Һ�У�c��H+����0.12 mol•L-1 | |
| D�� | ��0.1 mol•L-1��Na2A��Һ�У�c��A2-��+c��HA-��+c��Na+��=0.3mol•L-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��ӦA��g��+B��g��?C��g��+D��g�������е������仯��ͼ��ʾ���ش���������
��ӦA��g��+B��g��?C��g��+D��g�������е������仯��ͼ��ʾ���ش����������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢� | B�� | �ڢ� | C�� | �ڢ� | D�� | �٢� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����AgNO3��Һ��ϡ���ᣬ���ɰ�ɫ��������ȷ����Cl-���� | |
| B�� | �ýྻ��˿պȡ����Һ�ڻ��������գ�������ɫ���棬��ԭ��Һ��һ���������� | |
| C�� | �������ᣬ���ɵ�������ʹ����ʯ��ˮ����ǣ���ԭ��Һ��һ������CO32- | |
| D�� | ����KSCN��Һ����Һ����죬�ټ���ˮ����Һ�Ժ�ɫ����ԭ��Һһ����Fe2+ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com