
| 1 |
| 2 |
| 1 |
| 2 |
| 1 |
| 2 |
| 1 |
| 2 |
| A��X��y��Z |
| B��X��Z��Y |
| C��Y��X��Z |
| D��Z��X��Y |
| 1 |
| 2 |
| 1 |
| 2 |
| 1 |
| 2 |
| 1 |
| 2 |
| 1 |
| 2 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��C2H2(g)+H2(g)![]() C2H4(g)
C2H4(g)
��CH4(g)![]() H2(g)+
H2(g)+![]() C2H4(g)
C2H4(g)
��C(s)+2H2(g)![]() CH4(g)����H=-X kJ��mol-1
CH4(g)����H=-X kJ��mol-1
��C(s)+![]() H2(g)
H2(g)![]()
![]() C2H2(g)����H=-YkJ��mol-1
C2H2(g)����H=-YkJ��mol-1
��C(s)+H2(g)![]()
![]() C2H4(g)����H=-ZkJ��mol-1
C2H4(g)����H=-ZkJ��mol-1
���¶��½�ʱ��ʽƽ�������ƶ�����ʽƽ�������ƶ����ݴ��ж��ۡ���ʽ�й���X��Y��Z�Ĵ�С˳��������ȷ����( )
A.X��Y��Z B.X��Z��Y C.Y��X��Z D.Z��X��Y
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��C2H2(g)+H2(g)![]() C2H4(g)
C2H4(g)
��CH4(g)![]() H2(g)+1��
H2(g)+1��
��C(s)+2H2(g)![]() CH4(g)����H=-XkJ��mol-1
CH4(g)����H=-XkJ��mol-1
��C(s)+1��2H2(g)![]() 1��
1��
��C(s)+H2(g)![]() 1��
1��
���¶��½�ʱ��ʽƽ�������ƶ�����ʽƽ�������ƶ����ݴ��ж��ۡ���ʽ�й���X��Y��Z�Ĵ�С˳��������ȷ����( )
A.X��r��Z B.X��Z��Y C.Y��X��Z D.Z��X��Y
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��0103 �¿��� ���ͣ�������ѡ����
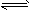 C2H4��g��
C2H4��g�� 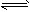 H2��g��+1/2C2H4��g��
H2��g��+1/2C2H4��g��  CH4��g������H= -X kJ��mol-1
CH4��g������H= -X kJ��mol-1 1/2C2H2��g������H= -Y kJ��mol-1
1/2C2H2��g������H= -Y kJ��mol-1 1/2C2H4��g����H= -ZkJ��mol-1
1/2C2H4��g����H= -ZkJ��mol-1 �鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����������ѧ����ʽ��
�� C2H2(g) + H2(g) ![]() C2H4(g)�� �� CH4(g)
C2H4(g)�� �� CH4(g) ![]() H2(g) + C2H4(g)��
H2(g) + C2H4(g)��
�� C(s) + 2H2(g) ![]() CH4(g)����H����x KJ�� mol��1��
CH4(g)����H����x KJ�� mol��1��
�� C(s) + H2(g)![]() 1/2C2H2(g)����H����y KJ�� mol��1��
1/2C2H2(g)����H����y KJ�� mol��1��
�� C(s) + H2(��)��1/2C2H4(g)����H����z KJ�� mol��1
���¶��½�ʱ��ʽƽ�������ƶ�����ʽƽ�������ƶ����ݴ��ж�������ʽ��x��y��z�Ĵ�С˳����ȷ����( )
A��x >y>z B��x>z>y C��y>x>z D��z
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com