
���� ��1�������θ�дΪ���������ʽ��д�����������д������������дˮ��
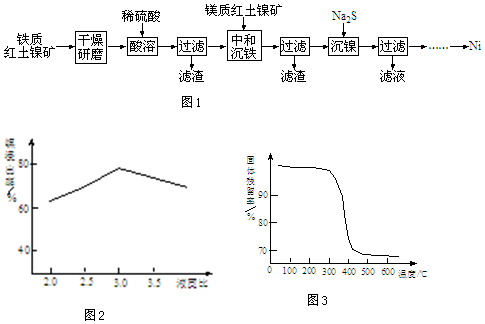
��2������n��H2SO4�����䣬��Һ�����ԽС������Ũ��Խ���ͼ������
��3������Fe��OH��3����������ԣ�
��4���ٸ��ݻ�ѧƽ�ⳣ������ɵã�
�ڸ���Ӱ�컯ѧƽ������ط�����
��5��Mg��OH��2���нϺ���ȼ�Ե�ԭ���ǣ���Mg��OH��2����ȼ�ҷֽ����ȣ������ɵ�H2O�������ȣ���ˮ�������������������ɵ�MgO����ȼ���۵�ߣ������ڿ�ȼ����棻�ܾ�ͼ��֪���¶Ƚϸ�ʱ��������þ�ֽ⳹�ף�
��� �⣺��1��������ʯMg6[Si4O10]��OH��8�������������ʽ�ɱ�ʾΪ3MgO•2SiO2•2H2O��
�ʴ�Ϊ��3MgO•2SiO2•2H2O��
��2������n��H2SO4�����䣬��Һ�����ԽС������Ũ��Խ��Һ�̱�С��3.0ʱ������ԽŨ��������ԽС������Ũ��Խ����Һճ����Խ������������ɢ��������Ӧ��
�ʴ�Ϊ��c��
��3��Fe��OH��3������������ԣ������������ӣ�
�ʴ�Ϊ��Fe��OH��3������������ԣ�
��4���ٷ�Ӧ3Fe3++Na++2SO42-+6H2O?Na��+6H+��H��0��ƽ�ⳣ��K=$\frac{{c}^{6}��{H}^{+}��}{{c}^{3}��F{e}^{3+}��•c��N{a}^{+}��•{c}^{2}��S{{O}_{4}}^{2-}��}$��
�ʴ�Ϊ��$\frac{{c}^{6}��{H}^{+}��}{{c}^{3}��F{e}^{3+}��•c��N{a}^{+}��•{c}^{2}��S{{O}_{4}}^{2-}��}$��
��Ϊ��߳���Ч����Ҫ���ƽ�������ƶ����÷�Ӧ�Ǹ����ȷ�Ӧ���ʿ��ʵ������¶ȣ��ӷ�Ӧ���������������Һ��Ũ�ȣ��Ӳ�������ʵ������Һ��pH��С������Ũ�ȣ������ڷ�Ӧ��������У�
�ʴ�Ϊ���ʵ������¶ȡ��ʵ������ҺpH���ʵ�����Na2SO4��Һ��Ũ�ȣ�
��5��Mg��OH��2���нϺ���ȼ�Ե�ԭ���ǣ���ͼ��֪�����¶Ƚϸ�ʱ�������������٣�˵������Mg��OH��2����ȼ�ҷֽ����ȣ������ɵ�H2O�������ȣ���ˮ�������������������ɵ�MgO����ȼ���۵�ߣ������ڿ�ȼ����棻��Mg��OH��2�ֽ��¶����ˣ�
�ʴ�Ϊ��Mg��OH��2�ֽ��¶����ˣ�
���� ���⿼�������ʵ��Ʊ����Ǹ߿��������ͣ��ؼ������˶�ͼ������Ŀ��飬��Ŀ�ѶȽϴ�Ҫ��ѧ���Ի���֪ʶ��������ã�����Ŀ��Ϣ���������ܸ������ʵ����ۣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | O2�ǻ�ԭ�� | B�� | NH3�������� | C�� | O2ʧȥ���� | D�� | NH3����������Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ˮ�������c��OH-�����٣��ڣ��ܣ��� | |
| B�� | �ٺ͢ۻ�Ϻ���Һ�����ԣ�c��Na+��+c��H+����c��CH3COO-��+c��Cl-�� | |
| C�� | �ٺܻ͢�Ϻ���Һ�����ԣ�$\frac{c��N{a}^{+}��}{c��C{H}_{3}CO{O}^{-}��}$=1 | |
| D�� | �ۺֱܷ͢�ϡ��100�����pH�ܣ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����е���֬ | B�� | �����еĵ��� | C�� | �߲��е���ά�� | D�� | �����еĵ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NH4CuSO3��ֻ��CuԪ�ر����� | |
| B�� | ��Ӧ�������������� | |
| C�� | �̼�����ζ�������Ƕ������� | |
| D�� | 1 mol NH4CuSO3��ȫ��Ӧת��0.5 mol���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
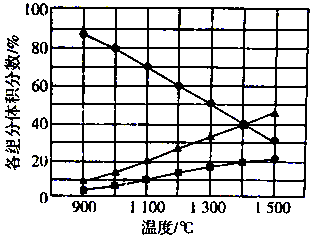 H2S�ڸ����·ֽ�������������S2����H2����ͬ�¶��£���Ӧ��ϵ�и���ֵ����������ͼ��ʾ����1400��ʱ��Ӧ��ϵ�л�������ƽ��Ħ������Ϊ��������
H2S�ڸ����·ֽ�������������S2����H2����ͬ�¶��£���Ӧ��ϵ�и���ֵ����������ͼ��ʾ����1400��ʱ��Ӧ��ϵ�л�������ƽ��Ħ������Ϊ��������| A�� | 20.8g/mol | B�� | 27.2 g/mol | C�� | 33.3 g/mol | D�� | 39.6 g/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com