
| �ζ����� | ��Ʒ������/g | KMnO4��Һ�����/mL | |
| �ζ�ǰ�̶�/mL | �ζ���̶�/mL | ||
| 1 | 0.300 0 | 1.02 | 24.04 |
| 2 | 0.300 0 | 2.00 | 25.03 |
| 3 | 0.300 0 | 0.20 | 23.24 |
 n��KMnO4����
n��KMnO4���� ��0.020 0 mol��L��1��23.03 mL��10��3 L��mL��1��1.151 5��10��3 mol
��0.020 0 mol��L��1��23.03 mL��10��3 L��mL��1��1.151 5��10��3 mol ��100%��82.91%��
��100%��82.91%��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

 ������ƿ�У���0.05mol/L������Һ���еζ������ı���Һ20.00ml����ش��������⣺
������ƿ�У���0.05mol/L������Һ���еζ������ı���Һ20.00ml����ش��������⣺
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
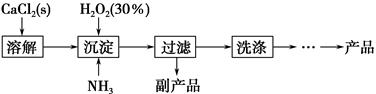
| A��ͼ1��ʾװ�ÿ��Ʊ����� |
| B��ͼ2��ʾװ�ÿɷ���CH3CH2OH��CH3COOC2H5�Ļ��Һ |
| C��ͼ3��ʾװ�ÿ��Ʊ����ռ���ϩ����֤���ױ����� |
| D��ͼ4��ʾװ�ÿ��Ʊ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
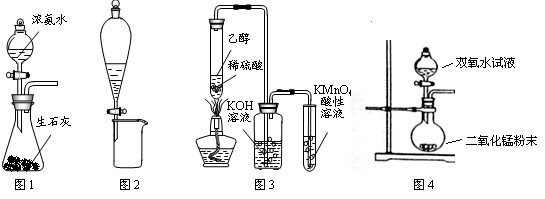
| A��������������� |
| B���������ƺ�Ũ�������ɶ������� |
| C��̼��ƺ��������ɶ�����̼ |
| D��Ũ��ˮ���ռ���ȡ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| A���Թ� | B�������� | C����ƿ | D������ |
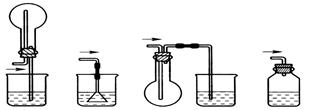
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

| A��a��b��c��d��e��f��g��h |
| B��a��e��d��c��b��h��i��g |
| C��a��d��e��c��b��h��i��g |
| D��a��c��b��d��e��h��i��f |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

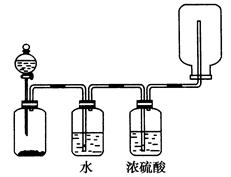
| A����AΪŨ����,BΪMnO2,C��ʢƷ����Һ,��C����Һ��ɫ |
| B����AΪ����,BΪ����,C��ʢ�Ȼ�����Һ,��C����Һ����� |
| C����AΪŨ��ˮ,BΪ��ʯ��,C��ʢAlCl3��Һ,��C���Ȳ�����ɫ����������ܽ� |
| D��ʵ������D������ֹ��Һ���������� |
�鿴�𰸺ͽ���>>
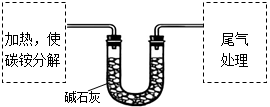
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
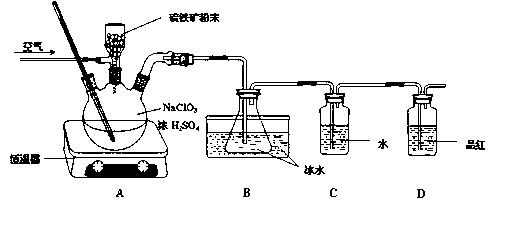
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����


�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com