
��8�֣���1��ʵ�����Ʊ�NH3�Ļ�ѧ����ʽΪ ���ɲ��� ���ռ���
![]()
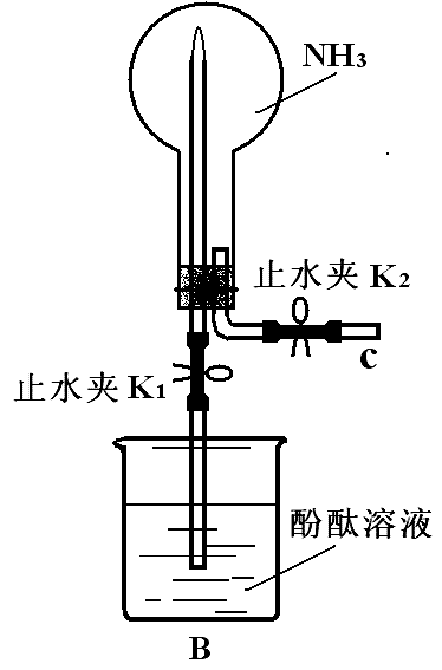
��2����ͬѧ����ͼBװ����NH3 ��Ȫʵ�飬�ر�K2 ����K1 ����������Բ����ƿ��һ��ʱ�����ƿ���к�ɫ��Ȫ����
![]() ��������γ���Ȫ��ԭ�� ��
��������γ���Ȫ��ԭ�� ��
![]() ���÷���ʽ��ʾ��̪����ԭ�� ��
���÷���ʽ��ʾ��̪����ԭ�� ��
![]() ��3����ͬѧ��Bװ����NH3��Cl2��Ӧ��ʵ�顣
��3����ͬѧ��Bװ����NH3��Cl2��Ӧ��ʵ�顣
����1���ر�K1����K2ͨ��Cl2 ����ƿ�г��ְ��̣�д����Ӧ�Ļ�ѧ����ʽ
����2��ͨ��Cl2��ǡ����ȫ��Ӧ�ر�K2 ����K1 ����ƿ�е������ǣ� ��ʵ����Ϻ���ƿ����Һ�����ռ��ƿ�ݻ��ķ����ǣ� ��
 ��ʦ����ָ���ο�ʱϵ�д�
��ʦ����ָ���ο�ʱϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 I���±���ʵ�����Ʊ�������й����ݣ�
I���±���ʵ�����Ʊ�������й����ݣ�| ��� | ʵ������ | ʵ��ԭ�� | ����װ�� |
| �� | ������ | H2O2��O2 | |
| �� | �ư��� | NH4Cl��NH3 | |
| �� | ������ | HCl��Cl2 |
| ||
| ||


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ������ʡ�����и����������ʼ죨���ۣ���ѧ���� ���ͣ������
�±���ʵ�����Ʊ�������й����ݣ�

��1�����������У����Ʊ����̿�������ѡ����ʵ�����������ʵ�ֵ��� ��������Ļ�ѧʽ�����ӷ�Ӧԭ���������Բ�ͬ����������������� ��������Ļ�ѧʽ�������ռ�����������ֻ����һ�ַ����ռ����� ��������Ļ�ѧʽ����
��2�����ݱ�������ʵ��ԭ����������װ����ѡ����ʵ����巢��װ�ã�����ѡ�����������Ӧ��ŵĿո��С�

��3�����������Ʊ�NH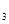 ��װ���Ʊ�O
��װ���Ʊ�O ����ѡ����Լ�Ϊ ���ѧʽ�������������Ʊ�O
����ѡ����Լ�Ϊ ���ѧʽ�������������Ʊ�O ��װ���Ʊ�NH
��װ���Ʊ�NH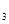 ����ѡ����Լ�������Ϊ ��
����ѡ����Լ�������Ϊ ��
��4�������ſ������ռ�Cl ���뻭�������ռ�װ��ͼ ��
���뻭�������ռ�װ��ͼ ��
��5�������������ת����ϵ����֪B��Y���������������е����֡�
a.��wΪMnO ʱ����Ӧ�ٲ����Ⱦ��ܷ�Ӧ����Ӧ�ܼ��Ȳ���˳�����У�д����Ӧ�ٵĻ�ѧ����ʽ
��
ʱ����Ӧ�ٲ����Ⱦ��ܷ�Ӧ����Ӧ�ܼ��Ȳ���˳�����У�д����Ӧ�ٵĻ�ѧ����ʽ
��
b.��wΪKMnO ʱ����Ӧ�ټ��Ȳ��ܽ��У���Ӧ�ܲ����Ⱦ��ܽ��У�д����Ӧ�ܵ����ӷ���ʽ��
��
ʱ����Ӧ�ټ��Ȳ��ܽ��У���Ӧ�ܲ����Ⱦ��ܽ��У�д����Ӧ�ܵ����ӷ���ʽ��
��
��6����֪ ������100mL��
������100mL�� ��Һ��ͨ���״����Cl
��Һ��ͨ���״����Cl 3.36L����ַ�Ӧ�����Һ��
3.36L����ַ�Ӧ�����Һ��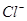 ��
�� �����ʵ���Ũ����ȣ���ͨ��Cl
�����ʵ���Ũ����ȣ���ͨ��Cl ǰ����Һ������䣩����ԭ
ǰ����Һ������䣩����ԭ ��Һ�����ʵ����ʵ���Ũ��Ϊ ��
��Һ�����ʵ����ʵ���Ũ��Ϊ ��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ��ɽ��ʡ�����и����ڶ���ģ�⿼�ԣ����ۣ���ѧ���� ���ͣ�ʵ����
��15�֣��ߴ�������������Fe2O3�����ִ����ӹ�ҵ����Ҫ���ϡ�ʵ��������������������Ҫ�ɷ�ΪFe2O3��FeO��������SiO2�����ʣ�Ϊԭ���Ʊ��ߴ��������IJ������£�

�ش��������⣺
 ��1������ʵ�����漰�ķ�Ӧ�У���һ����Ӧ�����ڻ��Ϸ�Ӧ��������������ԭ��Ӧ��д���÷�Ӧ�����ӷ���ʽ��
��
��1������ʵ�����漰�ķ�Ӧ�У���һ����Ӧ�����ڻ��Ϸ�Ӧ��������������ԭ��Ӧ��д���÷�Ӧ�����ӷ���ʽ��
��
��2��ʵ��������18.4mol��L-1��Ũ��������100mL 5.0mol��L-1������
��Һ�����õIJ���������ͷ�ιܡ���Ͳ���ձ����������⣬����
����д�������ƣ���
��3��ijͬѧ����ͼ��ʾװ�ý��й��˲�����
����ָ�����еĴ���֮���� ��
�ڹ��˺�ϴ�ӹ����������������ķ����� ��
��4��ijͬѧ����ͼ��ʾװ�ã�β������װ��δ������ʵ������ҺY��ͨ��NH3��CO2

������Ϊʵ�����Ʊ�NH3��CO2�ı�ѡҩƷ��
a.NH4Cl b.CaCO3����״�� c.Ca��OH��2 d.NaOH
e.Ũ��ˮ f.ϡ���� g.ϡ����
������װ��A�����Թ�������ҩƷ�����ѡ��Ϊ �� ����ҩƷ�����գ���װ��D��ҩƷ�����ѡ��Ϊ �� ����ҩƷ�����գ���
�����и����Ʊ�ʵ���У�Ҳ������װ��D��������ɵ��� ������ţ���
A��MnO2��Ũ���ᷴӦ�Ʊ�Cl2
B��Cu��Ũ���ᷴӦ����SO2
C����KMnO4�ֽ���O2
D���Ҵ������ᷴӦ�Ʊ���������
E��Zn��ϡ���ᷴӦ�Ʊ�H2
��д������װ��A�����Թ�����������Ӧ�Ļ�ѧ����ʽ ��
����ͨ��һ������NH3��CO2��װ��C������Һ��ֻ����S��N��H��O����Ԫ�ء���pH��ֽ�ⶨ����ҺpH�ķ����� ��������Һ�����ԣ�����Һ�е�NH+4��SO2-4�����ʵ���Ũ�ȼ��������ϵΪ �������ӵ�Ũ���÷���[NH+4]��[SO2-4]��ʾ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 I���±���ʵ�����Ʊ�������й����ݣ�
I���±���ʵ�����Ʊ�������й����ݣ�| ��� | ʵ������ | ʵ��ԭ�� | ����װ�� |
| �� | ������ | H2O2��O2 | |
| �� | �ư��� | NH4Cl��NH3 | |
| �� | ������ | HCl��Cl2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijУ��ѧ����С����װ��������������ͼ��ʾ�������������ӣ��Ʊ���ѧ��ѧ�м��ֳ������塣
����д���б����еĿհף�
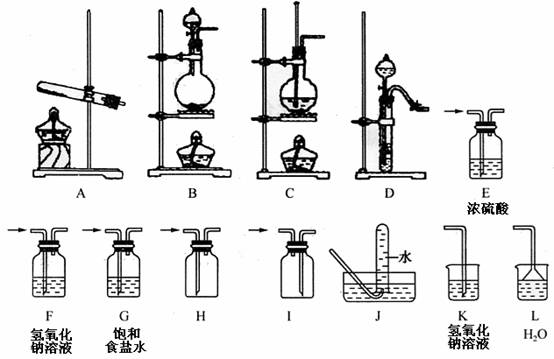
| ��� | ���� | װ�õ�����˳���ñ�ű�ʾ�� | ��Ҫ�����Լ����� | ʵ�����Ʊ�������Ļ�ѧ��Ӧ����ʽ�������ӷ�Ӧ��Ҫд�����ӷ�Ӧ����ʽ�� |
| ��1�� | CO2 | �Ʊ����ռ� �� ������ �� | ||
| ��2�� | NH3 | �Ʊ����ռ���β������ �� ������ ������ �� | ������NH3��ѡ�õ��Լ�
| |
| ��3�� | Cl2 | �Ʊ���������������ռ���β������ �� ������ ������ �� ���� ������ �� | ����β�����Լ�
|
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com