
���� ��һ�ݣ�����AgNO3��Һ�г���������˵����Һ�п��ܴ���Cl-��CO32-��SO42-��
�ڶ��ݣ�������NaOH��Һ�������ɵ�0.08mol����Ϊ��������Һ��һ������NH4+���������ʵ���Ϊ0.08mol��
�����ݣ��ܹ����Ȼ������ɰ�ɫ������Ϊ̼������ӻ���������ӣ�����������Ϣ��֪4.66gΪ���ᱵ��12.54gΪ���ᱵ��̼�ᱵ�ݴ˼����̼������Ӻ���������ӵ����ʵ������ٸ��ݵ���غ㣬�ó��Ƿ���ڼ����ӣ�
��� �⣺��һ�ݣ���һ�ݼ���AgNO3����Һ�г���������˵����Һ�п��ܴ��ڣ�Cl-��CO32-��SO42-��
�ڶ��ݣ�������NaOH��Һ���Ⱥ����ɵ�0.08mol����Ϊ����������Һ��һ������NH4+�������ʵ���Ϊ0.08mol��
�����ݣ�����������Ϣ��֪4.66Ϊ���ᱵ��n��BaSO4��=n��SO42-��=$\frac{4.66g}{233g/mol}$=0.02mol��12.54gΪ���ᱵ��̼�ᱵ������̼�ᱵ�����ʵ���Ϊ��$\frac{12.54g-4.66g}{197g/mol}$=0.04mol���ٸ��ݵ���غ㣬�����Ϊ��n��+��=n��NH4+��=0.08mol��n��-��=2n��CO32-��+2n��SO42-��=0.12mol����һ����K+������0.04mol��
��1������̼������ӡ���������Ӷ��ܹ��������ӷ�Ӧ����̼������������������������ȷ��ԭ��Һ���Ƿ���������ӣ�
�ʴ�Ϊ�����ܣ�
��2��������NaOH��Һ���Ⱥ��ռ���0.08mol���壬������Ϊ������˵����Һ�� һ����������ӣ�����ӵ����ʵ���Ũ��Ϊ��c��NH4+��=$\frac{0.08mol}{0.1L}$=0.8mol/L��
�ʴ�Ϊ��NH4+��0.8mol/L��
��3��������BaCl2��Һ�õ��������12.54g������������ϴ�ӡ������������Ϊ4.66g��˵������Ϊ���ᱵ��̼�ᱵ�Ļ�������4.66gΪ���ᱵ������n��BaSO4��=n��SO42-��=$\frac{4.66g}{233g/mol}$=0.02mol��̼�ᱵ����������Ϊ��12.54g-4.66g=7.88g������n��BaCO3��=n��CO32- ��=$\frac{7.88g}{197g/mol}$=0.04mol��
�ʴ�Ϊ��BaCO3��0.04 mol��BaSO4��0.02 mol��
��4���������Ϸ�����֪����Һ��һ�����ڣ�K+��NH4+��CO32-��SO42-�����ܺ���Cl-�������������ӣ������ӵ����ʵ�������0.04mol���������������ӣ������ӵ����ʵ���Ϊ0.04mol������A��ȷ��
��ѡA��
���� ���⿼���˳������������ӵļ��鷽������Ŀ�Ѷ��еȣ������漰�����ݵ���غ��ƶ����ӵĴ��ڣ�ע���������ճ������ӵļ��鷽��������������ѧ�����Ӧ����ѧ֪ʶ��������


 �����ƻ���ĩ��̶�100��ϵ�д�
�����ƻ���ĩ��̶�100��ϵ�д� �ܿ���ȫ��100��ϵ�д�
�ܿ���ȫ��100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ��� | ��ѧ��Ӧ | ���ӷ���ʽ | ���� |
| A | ��MgSO4��Һ����Ba��OH��2��Һ | Mg2++2OH-�TMg��OH��2�� | ��ȷ |
| B | ����ͭ��ϡ���ᷴӦ | CuO+2H+ $\frac{\underline{\;\;��\;\;}}{\;}$Cu2++H2O | ��ȷ |
| C | ��FeCl2��Һ��ͨ������ | Fe2++Cl2�TFe3++2Cl- | ��ȷ |
| D | AlCl3��Һ�м�������ˮ | Al3++3OH-�TAl��OH��3�� | ��ȷ |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ֻ�Т٢� | B�� | ֻ�Т٢ڢۢ� | C�� | ֻ�Т٢ڢ� | D�� | ȫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | O2��ֻ���ڷǼ��Թ��ۼ���SO2�ǹ��ۻ����� | |
| B�� | ��������O2����ԭ����ֻ��Fe2O3 | |
| C�� | ÿ����22.4LO2����ת��4NA������ | |
| D�� | SO2����ˮ֮�����Һ���Ե��磬����SO2�ǵ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Y�����ڱ��е�λ���ǵ�4���ڢ�B�� | |
| B�� | ���ڷǽ���Ԫ�� | |
| C�� | ${\;}_{39}^{89}$Y��${\;}_{39}^{89}$Y�����ֲ�ͬ�ĺ��� | |
| D�� | ����${\;}_{39}^{89}$Y����������������֮��Ϊ50 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

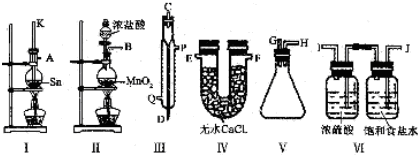
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���³�ѹ�£�2.24LH2O�з��ӵ���ĿΪ0.1NA | |
| B�� | 0.1mol•L-1NH4Cl��Һ�к���Cl-����ĿΪ0.1NA | |
| C�� | �����£�1.7gNH3�к�����ԭ����ĿΪ0.3 NA | |
| D�� | 5.6g�����������������г��ȼ�գ�ת�Ƶ�����Ϊ0.2NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��$\frac{3m}{2}$+$\frac{n}{2}$+2p��mol | B�� | ��3p-$\frac{3m}{2}$-$\frac{n}{2}$��mol | C�� | 3pmol | D�� | ��$\frac{3m}{2}$+$\frac{n}{2}$��mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
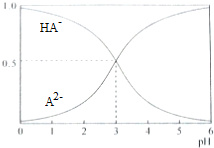 �����£�1mol/L��ij��Ԫ��H2A��Һ�У����ܴ��ڵĺ�A���ӣ�H2A��HA-��A2-�������ʵ���������x����pH�仯�Ĺ�ϵ��ͼ��ʾ������˵����ȷ���ǣ�������
�����£�1mol/L��ij��Ԫ��H2A��Һ�У����ܴ��ڵĺ�A���ӣ�H2A��HA-��A2-�������ʵ���������x����pH�仯�Ĺ�ϵ��ͼ��ʾ������˵����ȷ���ǣ�������| A�� | H2A�ĵ��뷽��ʽΪH2A?H+HA- | |
| B�� | ��pH=2��NaHA��Na2A�����Һ�м�ˮϡ��10������Һ��pH=3 | |
| C�� | ����ͬ���ʵ�����NaHA��Na2A��������ˮ���û����Һ��pHһ��Ϊ3 | |
| D�� | Na2A��Һ���ڣ�c��0H-��=c��H+��+c��HA-�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com