
���������������������ˮ��ҵ��ˮ����������������������Ͽ졣ʵ���ҿ��ö�������Ϊ��Ҫԭ���Ʊ�������ء��䲿���������£�
(1)�ڢٲ��в��������������ô�������ԭ����(�û�ѧ����ʽ��ʾ)________________________________________��
(2)KOH��KClO3��MnO2���۷�Ӧ����ī��ɫK2MnO4�Ļ�ѧ����ʽΪ________________________________________��
(3)�ڢܲ�ͨ��CO2����ʹMnO42��������Ӧ������MnO4����MnO2����K2MnO4��ȫ��Ӧʱ��ת��ΪKMnO4�İٷ���ԼΪ________(��ȷ��0.1%)��
(4)�ڢݲ����ȹ��˵�Ŀ����______________________________________��
(5)�ڢ�����Ũ����Һ����ϸС��������ʱ��ֹͣ���ȣ���ȴ�ᾧ��________��ϴ�ӡ������������У��¶Ȳ��˹��ߣ���Ϊ____________________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���ȵ�ϡ�������ܽ���11.4 g FeSO4���壬������50 mL 0.5 mol��L��1 KNO3��Һʱ�����е�Fe2��ȫ��ת����Fe3����KNO3Ҳ��ȫ��Ӧ���ų�NxOy���塣
(1)�����x�� ��y�� ��
(2)��ƽ�÷�Ӧ�ķ���ʽ��
FeSO4�� KNO3�� H2SO4= K2SO4�� Fe2(SO4)3��  (NxOy)�� H2O(��ƽʱx��y�þ�����ֵ��ʾ����������
(NxOy)�� H2O(��ƽʱx��y�þ�����ֵ��ʾ���������� ��)��
��)��
(3)��Ӧ������������ ��
(4)��˫���ŷ���ʾ�÷�Ӧ�еĵ���ת�Ʒ������Ŀ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����һ�ֻ��ý����������ܶ�С���۵�ߡ�������ǿ����еǿ�ȸߵ����ܡ���ҵ�ϳ�������ֽ����ѿ�ʯ���Ʊ��������ѣ�����ұ���ѣ���Ҫ�����������Ӧ��
��FeTiO3��2H2SO4=TiOSO4��FeSO4��2H2O
��TiOSO4��2H2O=H2TiO3����H2SO4
��H2TiO3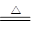 TiO2��H2O
TiO2��H2O
��TiO2��2C��2Cl2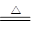 TiCl4����2CO��
TiCl4����2CO��
��TiCl4��2Mg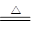 2MgCl2��Ti
2MgCl2��Ti
(1)������������Ӧ�����������������________��
A����Ӧ���Ƿ�������ԭ��Ӧ
B����Ӧ��������������
C����Ӧ���е�TiO2��������
D����Ӧ�ݱ����˽���þ�Ƚ����ѵĻ�ԭ��ǿ
(2)�Ѿ��к�ǿ����ʴ�ԣ����¶���ԭ��ķ�����ȷ����________��
A��������𡢲�һ���IJ����ý���
B�������ѵı������γ����ܵ�����Ĥ
C��������������ȸ�ʴ������Ӧ
D�����кܸߵ�ǿ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
Cu��һ��Ũ�ȵ�HNO3��ӦΪ:3Cu+2NO3��+xH+ 3Cu2++2R+yH2O��
3Cu2++2R+yH2O��
(1)��Ӧ�е�x=����������������
(2)��Ӧ����R�Ļ�ѧʽΪ����������������
(3)�μӷ�Ӧ��Cu������HNO3�����ʵ���֮��Ϊ������������
(4)1.5 mol Cu��ȫ��Ӧʱת�Ƶĵ�����Ϊ��������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������(K2FeO4)���к�ǿ�������ԣ����������������й㷺Ӧ�á�
��1��K2FeO4��������Чˮ�����������������÷ֱ��ǣߣߣߣߣߡ�
[��֪��FeO42����3e����4H2O Fe(OH)3��5OH��]
Fe(OH)3��5OH��]
��2���Ʊ�K2FeO4���Բ���ʪʽ����������������ͼ��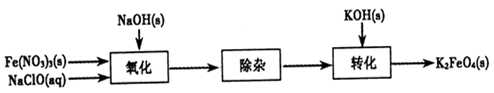
�����������������ӷ���ʽ��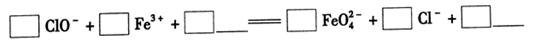
�ڳ��ӹ���Ŀ���dz�ȥNa��FeO4��Һ�е����ʣ���ȥ��������Ҫ�Уߣߣߣߣ�(д��ѧʽ)��
��ת�����������ij�¶��½��У�����¶����ܽ�ȣ�Na��FeO4�ߣߣߣߣ�K2FeO4 (�����������������)��
��3��ʵ����ģ�ҵ��ⷨ��ȡK2FeO4��װ������ͼ��
�ٴ�װ���е�Դ�ĸ����ǣߣߣߣ�(�a����b��)��
�������ĵ缫��ӦʽΪ�ߣߣߣߣߣߡ�
��4����֪K2FeO4ϡ��Һ�д�������ƽ�⣺4FeO42����10H2O 4Fe(OH)3��8OH����3O2��ʵ����K2FeO4��ҺŨ�����¶Ⱥ�pH�Ĺ�ϵ�ֱ�����ͼ��ʾ��
4Fe(OH)3��8OH����3O2��ʵ����K2FeO4��ҺŨ�����¶Ⱥ�pH�Ĺ�ϵ�ֱ�����ͼ��ʾ��
����ͼI�ɵó��Ľ��ۣ��÷�Ӧ�ġ��ȣߣߣ�0(�����������������)��
��ͼ����pH���ߣߣ�pH3(�����������������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���÷Ͼɶ�п��Ƥ���Ʊ�����Fe3O4�������Ӽ�������ZnO���Ʊ�����ͼ���£�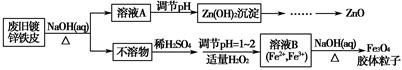
��֪��Zn���仯�����������Al���仯������������ơ���ش��������⣺
��1����NaOH��Һ�����Ͼɶ�п��Ƥ��������________��
| A��ȥ������ | B���ܽ��п�� | C��ȥ������ | D���ۻ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ʳ�Ρ�̼���ƺ�̼�������������г��������Ρ���ش��������⡣
��1��̼�����Ƶ�ˮ��Һ��__________�ԡ�����ᡱ��������С�������ȥ̼���ƹ����л��е�����̼�����ƣ���Ӧ�Ļ�ѧ����ʽΪ_________________��
��2����������̼���ƺ�̼�����Ʒֱ����������ᷴӦʱ���ɵ�CO2����ǰ��________���ߣ��>������<����=������
��3�����κ����������ʣ���ҪΪCaCl2��MgCl2��Na2SO4�ȣ����ô�����ȡ����ѧ��������NaCl������Ϊ�ܽ⡢�ӹ���a���ӹ���NaOH���ӹ���b�����ˡ����������ᣬ�����ᾧ�õ�����ѧ��������NaCl���塣�Լ�a��b�ֱ���________(����ţ�
A.Na2CO3 BaCl2 B.BaCl2 Na2CO3 C.BaCl2 Na2SO4
��4����ҵ���õ�ⱥ��ʳ��ˮ�ķ��������������ռ
��ij��������������й©�¼���������Ա����NaOH��Һ�γ�ҺĻ����Χ������й©���������䷴Ӧԭ��_________________________________�������ӷ���ʽ��ʾ����
�ڹ�ҵ�Ͽ��ð��������������Ĺܵ��Ƿ�©������Ӧ����ʽ���£� �÷�Ӧ�У�____________Ԫ�ر���ԭ���÷�Ӧ���������ͻ�ԭ�����ʵ���֮��Ϊ__________��
�÷�Ӧ�У�____________Ԫ�ر���ԭ���÷�Ӧ���������ͻ�ԭ�����ʵ���֮��Ϊ__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������ԭ��Ӧ�������������о��й㷺����;���ᴩ�Ž�
��1��ˮ���������Ҫ��ɳɷ֣��������к�������һ�����ʡ��������ֻ�����Ӧ������������ԭ��Ӧ�Ĺ�ϵ��Ҳ������ͼ���
��д����ˮ�μӵķ��Ϸ�Ӧ���͢���һ����ѧ����ʽ��________________________________��
����ˮΪ_______����
��2���Ȼ�麟��������ӡ��磺�ں���ͭ��ʱ���Ȼ�麟�ȥͭ�����������ͭ�Ա㺸�ӣ��䷴ӦΪ��
_____CuO+_____NH4Cl _____Cu+_____CuCl2+N2��+_____H2O��
_____Cu+_____CuCl2+N2��+_____H2O��
����ƽ��������ԭ��Ӧ����ʽ��
�ڸ÷�Ӧ�У���������Ԫ����_______����Ԫ�����ƣ�����������_______���ѧʽ����
��3��������뽹̿��ʯӢɰ��ϣ��ڵ�¯�м��ȵ�1 500 �����ɰ��ף���ӦΪ��2Ca3��PO4��2+6SiO2=6CaSiO3+P4O10��10C+P4O10=P4+10CO��ÿ����1 mol P4ʱ������_______mol���ӷ���ת�ơ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
Fe2����I�������ֳ����Ļ�ԭ�����ӡ�
��1����FeSO4��Һ�еμ���ˮ����Һ��dz��ɫ��ɻ�ɫ����Ӧ�����ӷ���ʽΪ________________________����KI��Һ�еμ���ˮ����Һ����ɫ��ɻ�ɫ����Ӧ�����ӷ���ʽΪ______________________��
��2������FeSO4��Һ��KI��Һ����ˮ��2% KSCNΪ�Լ�֤��I���Ļ�ԭ��ǿ��Fe2�������ʵ�鷽�����������ʵ�鲽�衢Ԥ������ͽ��ۡ�
| ʵ�鲽�� | Ԥ����������� |
| ����1��ȡ2mLFeSO4��Һ��2mLKI��Һ������Թ��У��ٵμ�1~2����ˮ�� | ������Һ��ɻ�ɫ�� ���ۣ� �� |
| ����2��__________________________ __________________________________ | ���� �� ���ۣ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com