
��ͼΪ����A��I��ת����ϵ�����ַ�Ӧ�������û���г���������BΪij���������Ҫ�ɷ֣�����һϵ�з�Ӧ�ɵõ�E��F��D��E������Ϊ���壬D��FΪ�������ʣ�����F����H��Ũ��Һ����ʱ������Ӧ��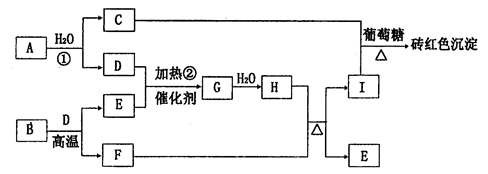
��ش��������⣺
��1��д��G�Ļ�ѧʽ��________________��
��2����Ӧ�ٵ����ӷ���ʽΪ__________________��
��3������F��H��Ũ��Һ������Ӧ�Ļ�ѧ����ʽΪ______________________��
��4����Pt���缫���I��Һ����ȫ����ĵ������Һ��_______________���������ĵ缫��ӦʽΪ____________________��
��5����֪ÿ16gE��D��ȫ��Ӧ���ų�24��75 kJ��������Ӧ�ڵ��Ȼ�ѧ����ʽΪ
________________________________________________��
��6����ҵ�Ʊ�Gʱ���������������Eռ7����Dռ11��������100������������뷴Ӧװ�ã�����������ָ���ԭ�¶Ⱥ�ѹǿ���Ϊ97��2�������E��ת����Ϊ________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
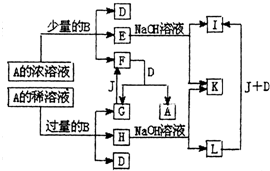 A��L�������ĸ���������ѧ��ѧ�ﳣ�������ʣ���֪B��J�ǵ��ʣ�A�ǻ����E����Һ��������Һ��ϣ���Һ����ɫ��A��L������֮�����Ӧת����ϵ����ͼ��ʾ����ش�
A��L�������ĸ���������ѧ��ѧ�ﳣ�������ʣ���֪B��J�ǵ��ʣ�A�ǻ����E����Һ��������Һ��ϣ���Һ����ɫ��A��L������֮�����Ӧת����ϵ����ͼ��ʾ����ش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(10�֣����ù��ܺ�������ɽ�CO2��H2O(g)ת��ΪCH4��O2�����������ʱ���ڲ�ͬ������I ,II��III)�����£�CH4�IJ��������ʱ��ı仯����ͼ��ʾ��
(1)��O〜30Сʱ�ڣ�CH4��ƽ������������
�ɴ�С��˳��Ϊ_________����Ӧ��ʼ���15Сʱ�ڣ��ڵ�_________�ִ����������£��ռ���CH4��ࡣ
(2) ������CH4��H2O(g)ͨ��۽�̫���ܷ�Ӧ����������Ӧ
CH4(g)+H2O(g)CO(g)+3H2(g) ��H=+206kJ��mol-1���������ʵ�����CH4��H2O(g)����1L�����ܱ�������ij�¶��·�Ӧ�ﵽƽ�⣬��ʱ���CO�����ʵ���ΪO.10 mol,CH4��ƽ��ת����Ϊ91 %������¶��¸÷�Ӧ��ƽ�ⳣ��Ϊ_________ (������ȡ��������
(3)�÷�Ӧ������CO��H2�������ϳɿ�������Դ�״�����֪CO(g)��CH3OH�ŵ�ȼ�����ֱ�Ϊ
��
����CH3OH(l)����ȫȼ������CO(g)��H2O(l)���Ȼ�ѧ����ʽΪ_________��
(4)��ҵ�ϳ����÷�ӦCO(g)+2H2(g) CH3OH (g), ��H<0�ϳɼ״�����230��C〜270��C��Ϊ������Ϊ�о��ϳ�������ʵ���ʼ��ɱ�n(H2)��n(C0),�ֱ���230��C��2500C��2700C����ʵ�飬�����ͼ��
��2700C��ʵ��������Ӧ��������_________(����ĸ����
��2300Cʱ����ҵ����������õĺϳ������n(H2):n(CO)�ı�ֵ��Χ��_________(����ĸ����
A. 1 〜1.5 B. 2. 5〜3 C. 3. 5〜4. 5
(5)ijͬѧ��ʯīΪ�缫����KOH��ҺΪ�������Ƽ״�ȼ�ϵ�أ��为���ĵ缫��ӦʽΪ_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(16��)
������λ�ڶ����ڵ��������ڡ���������ķǽ���Ԫ��X��Y����֪��Ԫ������������ˮ�����Ϊǿ�ᣮ������ͼת����ϵ����Ӧ���������ֲ�������ȥ�����ش��������⣺
��1����A��B��C��D��Ϊ��XԪ�صĻ������A��һ��������ֻ����10�����ӣ���
��A�ķ��ӹ���Ϊ ��
�ڷ�Ӧ��Ļ�ѧ����ʽΪ ��
�ۻ�����NaX3�Ǻϳɡ���ơ����м�������ʣ�NaX3��ײ��������Na3X����һ�����嵥�ʣ���д���÷�Ӧ�Ļ�ѧ����ʽ ��
��2����A��B��C��D��Ϊ��YԪ�صĻ������A��Ħ������Ϊ120 g��mol![]() ����
����
�ٷ�Ӧ������Һ�����������õľ������� ���壨����ӡ��������ӡ�����ԭ�ӡ�����
�ڷ�ӦI�Ļ�ѧ����ʽΪ ��
��������6gA������B����̬��ȫ��ת��ΪC����̬��ʱ�ų�9.83 KJ��������д�ڢ�Ӧ���Ȼ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010���������Ͽ���ѧ������ǰ��һ��ģ�⣨���ۣ���ѧ���� ���ͣ������
(16��)
������λ�ڶ����ڵ��������ڡ���������ķǽ���Ԫ��X��Y����֪��Ԫ������������ˮ�����Ϊǿ�ᣮ������ͼת����ϵ����Ӧ���������ֲ�������ȥ�����ش��������⣺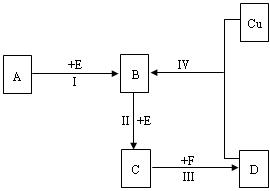
��1����A��B��C��D��Ϊ��XԪ�صĻ������A��һ��������ֻ����10�����ӣ���
��A�ķ��ӹ���Ϊ ��
�ڷ�Ӧ��Ļ�ѧ����ʽΪ ��
�ۻ�����NaX3�Ǻϳɡ���ơ����м�������ʣ�NaX3��ײ��������Na3X����һ�����嵥�ʣ���д���÷�Ӧ�Ļ�ѧ����ʽ ��
��2����A��B��C��D��Ϊ��YԪ�صĻ������A��Ħ������Ϊ120 g��mol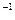 ����
����
�ٷ�Ӧ������Һ�����������õľ������� ���壨����ӡ��������ӡ�����ԭ�ӡ�����
�ڷ�ӦI�Ļ�ѧ����ʽΪ ��
��������6gA������B����̬��ȫ��ת��ΪC����̬��ʱ�ų�9.83 KJ��������д�ڢ�Ӧ���Ȼ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com