CO
2��SO
2��NO
x�ǶԻ���Ӱ��ϴ�����壬���ƺ�����CO
2��SO
2��NO
x�ǽ������ЧӦ����������⻯ѧ��������Ч;����
��1����Ч����̼�����ֶ�֮һ�ǽ��ܣ��������ⷽ��������ܵ���
��������ĸ��ţ�
A�����ˮ���⣺2H
2O
2H
2��+O
2��
B������ʹˮ�ֽ����⣺2H
2O
2H
2��+O
2��
C��̫������ֽ�ˮ���⣺2H
2O
2H
2��+O
2��
D����Ȼ�����⣺CH
4+H
2O
CO+3H
2��2��CO
2��ת�����л���ʵ��̼ѭ���������Ϊ1L���ܱ������У�����1mol CO
2��3mol H
2��һ�������·�����Ӧ��CO
2��g��+3H
2��g ��?CH
3OH��g��+H
2O��g����H=-49.0kJ/mol�����CO
2��CH
3OH��g����Ũ����ʱ��仯��ͼ��ʾ��

�ٷ�Ӧ���е�10minʱCO
2��ת����Ϊ
����4min��10min��v��H
2��=
mol?L
-1?min
-1��
����˵��������Ӧ�ﵽƽ��״̬����
������ĸ��ţ���
A��CO
2��CH
3OH�����ʵ���Ũ�����ʱ
B�����������ܶȲ���ʱ��ı仯���仯
C����λʱ����ÿ����3mol H
2��ͬʱ����1mol H
2O
D���������������ʵ������ֲ���
��3��NO
x������β���е���Ҫ��Ⱦ��֮һ����������������ʱ�������ͷ�Ӧ���������仯ʾ��ͼ���£�

��д���÷�Ӧ���Ȼ�ѧ����ʽ��
��
��ͨ��NO
x�������ɼ��NO
x�ĺ������乤��ԭ��ʾ��ͼ���£�

����������
ԭ�����ԭ��ء����ء����������ģ�Pt�缫�Ϸ�������
��Ӧ�����������ԭ������






 ���Ž�������С״Ԫϵ�д�
���Ž�������С״Ԫϵ�д�
 ��ѧ����С���������ͼ��ʾ��һ�����巢�����ռ���β������װ�ã���̽����װ�õĶ�����ܣ�
��ѧ����С���������ͼ��ʾ��һ�����巢�����ռ���β������װ�ã���̽����װ�õĶ�����ܣ� ��������A�ܷ�����ͼһϵ��ת����
��������A�ܷ�����ͼһϵ��ת����
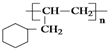 ����·������A������Ŀ����� �ڼ�����д����Ӧ�Լ�����Ӧ��������
����·������A������Ŀ����� �ڼ�����д����Ӧ�Լ�����Ӧ�������� ���������������ȵ������ҵ��µĴ�л�����ۺ�֢���Ը�Ѫ��Ϊ��Ҫ��־���������������ʳƷ��ȱ���˶�����������
���������������ȵ������ҵ��µĴ�л�����ۺ�֢���Ը�Ѫ��Ϊ��Ҫ��־���������������ʳƷ��ȱ���˶�����������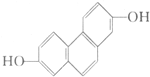 ���Ӻ˴Ź��������о��л�������ṹ�������ֶ�֮һ���ṹ�еĵ�Ч��ԭ�Ӻ˴Ź������ж���������Ӧ�ķ�ֵ ���źţ������з��ǿ����ṹ�е�Hԭ���������ȣ��Իش𣬽ṹ��ʽΪ��ͼ��ʾ���л������
���Ӻ˴Ź��������о��л�������ṹ�������ֶ�֮һ���ṹ�еĵ�Ч��ԭ�Ӻ˴Ź������ж���������Ӧ�ķ�ֵ ���źţ������з��ǿ����ṹ�е�Hԭ���������ȣ��Իش𣬽ṹ��ʽΪ��ͼ��ʾ���л������