
��֪N2(g)��3H2(g)![]() 2NH3(g)������һ�ܱ������г���1molN2��3molH2���ڸ��¡���ѹ�ʹ������������·�����Ӧ�������й�˵����ȷ���ǣ� ��
2NH3(g)������һ�ܱ������г���1molN2��3molH2���ڸ��¡���ѹ�ʹ������������·�����Ӧ�������й�˵����ȷ���ǣ� ��
A�����տ�������2mol NH3
B���ﵽ��ѧƽ��״̬ʱ������Ӧ���淴Ӧ�����ʶ�Ϊ0
C���ﵽ��ѧƽ��״̬ʱ��������N2��H2��NH3�����ʵ���֮��Ϊ1��3��2
D���ﵽ��ѧƽ��״̬ʱ��N2��H2��NH3�����ʵ���Ũ�Ȳ��ٱ仯
 ����ѧ��Ӯ�����ϵ�д�
����ѧ��Ӯ�����ϵ�д� ѧ���쳵�����ּ��������ҵ�½����������ϵ�д�
ѧ���쳵�����ּ��������ҵ�½����������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ��ѧ�� | N��N | H-O | N-H | O=O |
| ����/kJ?mol-1 | 945 | 463 | 391 | 498 |
 2NH3��g��+
2NH3��g��+| 3 |
| 2 |
 2NH3��g��+
2NH3��g��+| 3 |
| 2 |
| T/�� | 30 | 40 | 50 |
| ����NH3��/��10-6mol/L�� | 4.8 | 5.9 | 6.0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| T/K | 303 | 313 | 323 | 353 |
| NH3������/��10-6 mol�� | 4.8 | 5.9 | 6.0 | 2.0 |
| 3 |
| 2 |
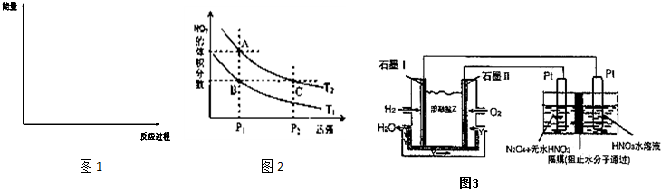
| 4 |
| 7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʱ�䣨h�� ���ʵ�����mol�� |
0 | 1 | 2 | 3 | 4 |
| N2 | 1.50 | n1 | 1.20 | n3 | n5 |
| H2 | 4.50 | 4.20 | 3.60 | n4 | n6 |
| NH3 | O | O��20 | n2 | 1��OO | 1��OO |
| A����Ӧ3h�ڣ���Ӧ������v��N2��ΪO.17 mol?L-1?h-1 |
| B�����¶��£��÷�Ӧ��ƽ�ⳣ��ΪO.037 |
| C����Ӧ���е�1Сʱʱ�ų�������Ϊ9.23 kJ |
| D��4hʱ�����ټ���1 molN2���ﵽ�µĻ�ѧƽ��ʱ��N2��ת������ԭ�������� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com