
ФГЬўЕФбмЩњЮяAЕФеєЦћУмЖШЪЧЯрЭЌЬѕМўЯТЧтЦјЕФ50БЖЃЌЦфжаЬМЁЂЧтдЊЫиЕФКЌСПЙВЮЊ68%ЃЌЦфгрЮЊбѕдЊЫиЁЃAжаЬМСДЮожЇСДЃЌ1 mol AФмгы2 mol H2ЗЂЩњМгГЩЗДгІЃЌЕЋВЛФмгыфхЫЎЗЂЩњМгГЩЗДгІЁЃИљОнЯТУцЕФзЊЛЏЙиЯЕЭъЛиД№ЯТСаЮЪЬтЁЃ
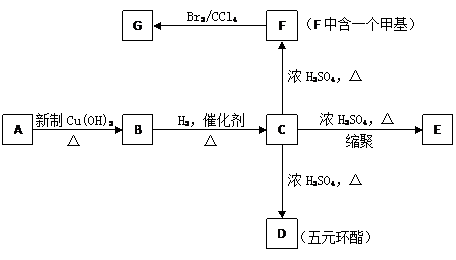
AЕФЗжзгЪНЮЊ
DЕФНсЙЙМђЪНЮЊ
аДГіЯТСазЊЛЏЕФЛЏбЇЗНГЬЪН
ЂйAЁњB
ЂкCЁњE
ЂлGгыNaOHШмвКЗДгІ
ЗжзгЪНгыFЯрЭЌЃЌОпгаЯТСааджЪЕФЭЌЗжвьЙЙЬхга 9 жж ЁЃ
ЂйФмЪЙфхЫЎЭЪЩЋ ЂкВЛФмгыNaHCO3ШмвКЗДгІ ЂлФмгыNaOHШмвКЗДгІ ЂмЗжзгНсЙЙЮожЇСД
аДГіЗћКЯЬѕМўЕФЭЌЗжвьЙЙЬхНсЙЙМђЪНЃЈШЮаДСНИіЃЉ
(1)C5H8O2(2Зж)

(2) (2Зж) ЃЈ3ЃЉ
![]() (2Зж)
(2Зж)
![]() (2Зж)
(2Зж)
(4) 9 (1Зж) CH3CH=CHCOOCH3 CH2=CHCOOCH2CH3 (4Зж)ЃЈЦфЫќКЯРэД№АИвВИјЗжЃЉ
ДЫЬтЕФЙиМќЪЧШЗЖЈAЕФНсЙЙЁЃЭЈЙ§МЦЫуПЩЭЦГіAЕФЗжзгЪНЮЊC5H8O2ЃЌФмгыаТжЦЧтбѕЛЏЭЗДгІПЩЭЦВтКЌ-CHOЁЃИљОнAжаЬМСДЮожЇСДЃЌ1 m![]() ol AФмгы2 mol H2ЗЂЩњМгГЩЗДгІЃЌЕЋВЛФмгыфхЫЎЗЂЩњМгГЩЗДгІЃЌПЩЭЦВтAжаЛЙКЌгавЛИієЪЛљЁЃдйИљОнCЁњDЩњГЩЕФЪЧЮхдЊЛЗѕЅЃЌМДПЩЭЦВтAЕФНсЙЙМђЪНЮЊ
ol AФмгы2 mol H2ЗЂЩњМгГЩЗДгІЃЌЕЋВЛФмгыфхЫЎЗЂЩњМгГЩЗДгІЃЌПЩЭЦВтAжаЛЙКЌгавЛИієЪЛљЁЃдйИљОнCЁњDЩњГЩЕФЪЧЮхдЊЛЗѕЅЃЌМДПЩЭЦВтAЕФНсЙЙМђЪНЮЊ ЁЃGгыNaOHШмвКЕФЗДгІжївЊЪЧвЊЭЌЪБПМТЧТБДњЬўЕФЫЎНтКЭєШЛљЕФжаКЭЁЃ
ЁЃGгыNaOHШмвКЕФЗДгІжївЊЪЧвЊЭЌЪБПМТЧТБДњЬўЕФЫЎНтКЭєШЛљЕФжаКЭЁЃ


 УПШе10ЗжжгПкЫуаФЫуЫйЫуЬьЬьСЗЯЕСаД№АИ
УПШе10ЗжжгПкЫуаФЫуЫйЫуЬьЬьСЗЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
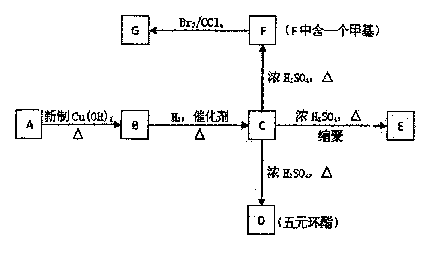 ФГЬўЕФбмЩњЮяAЕФеєЦћУмЖШЪЧЯрЭЌЬѕМўЯТЧтЦјЕФ50БЖЃЌЦфжаЬМЁЂЧтдЊЫиЕФКЌСПЙВЮЊ68ЃЅЃЌЦфгрЮЊбѕдЊЫиЁЃAжаЬМСДЮожЇСДЃЌ1 mol AФмгы2mol H2ЗЂЩњМгГЩЗДгІЃЌЕЋВЛФмгыфхЫЎЗЂЩњМгГЩЗДгІЁЃИљОнЯТУцЕФзЊЛЏЙиЯЕЛиД№ЯТСаЮЪЬтЁЃ
ФГЬўЕФбмЩњЮяAЕФеєЦћУмЖШЪЧЯрЭЌЬѕМўЯТЧтЦјЕФ50БЖЃЌЦфжаЬМЁЂЧтдЊЫиЕФКЌСПЙВЮЊ68ЃЅЃЌЦфгрЮЊбѕдЊЫиЁЃAжаЬМСДЮожЇСДЃЌ1 mol AФмгы2mol H2ЗЂЩњМгГЩЗДгІЃЌЕЋВЛФмгыфхЫЎЗЂЩњМгГЩЗДгІЁЃИљОнЯТУцЕФзЊЛЏЙиЯЕЛиД№ЯТСаЮЪЬтЁЃ
ЃЈ1ЃЉAЕФЗжзгЪНЮЊ ЃЛ
ЃЈ2ЃЉDЕФНсЙЙМђЪНЮЊ ЃЌDЕФКЫДХЙВеёЧтЦзЭМжаНЋГіЯж жжЗхЁЃ
ЃЈ3ЃЉGжаЫљКЌЙйФмЭХЕФУћГЦЮЊЃК
ЃЈ4ЃЉBЁЊCЕФЗДгІРраЭЪЧ ЃЛCЁњDЕФЗДгІРраЭ ЁЃ
aЃЎЯћШЅЗДгІ bЃЎМгГЩЗДгІcЃЎЛЙдЗДгІ dЃЎѕЅЛЏЗДгІ
ЃЈ5ЃЉаДГіЯТСазЊЛЏЕФЛЏбЇЗНГЬЪН
ЂйAЁњB
ЂкCЁњE
ЂлGгыNaOHШмвКЗДгІ
ЃЈ6ЃЉMЪЧFЕФЭЌЯЕЮяЃЌЦфЯрЖдЗжзгжЪСПБШFtJJЁЂ14ЃЌаДГіТњзуЬѕМўЕФMЕФЫљгаПЩФмЕФНсЙЙЃК
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃКЬьНђСљаЃ2010НьИпШ§ЕкШ§ДЮСЊПМ ЬтаЭЃКЬюПеЬт
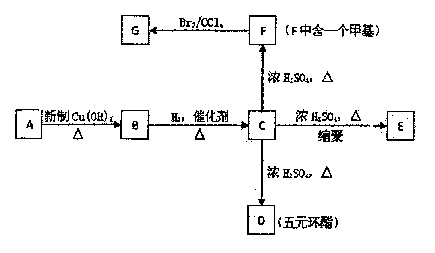 ФГЬўЕФбмЩњЮяAЕФеєЦћУмЖШЪЧЯрЭЌЬѕМўЯТЧтЦјЕФ50БЖЃЌЦфжаЬМЁЂЧтдЊЫиЕФКЌСПЙВЮЊ68ЃЅЃЌЦфгрЮЊбѕдЊЫиЁЃAжаЬМСДЮожЇСДЃЌ1 mol AФмгы2
mol H2ЗЂЩњМгГЩЗДгІЃЌЕЋВЛФмгыфхЫЎЗЂЩњМгГЩЗДгІЁЃИљОнЯТУцЕФзЊЛЏЙиЯЕЛиД№ЯТСаЮЪЬтЁЃ
ФГЬўЕФбмЩњЮяAЕФеєЦћУмЖШЪЧЯрЭЌЬѕМўЯТЧтЦјЕФ50БЖЃЌЦфжаЬМЁЂЧтдЊЫиЕФКЌСПЙВЮЊ68ЃЅЃЌЦфгрЮЊбѕдЊЫиЁЃAжаЬМСДЮожЇСДЃЌ1 mol AФмгы2
mol H2ЗЂЩњМгГЩЗДгІЃЌЕЋВЛФмгыфхЫЎЗЂЩњМгГЩЗДгІЁЃИљОнЯТУцЕФзЊЛЏЙиЯЕЛиД№ЯТСаЮЪЬтЁЃ
ЃЈ1ЃЉAЕФЗжзгЪНЮЊ ЃЛ
ЃЈ2ЃЉDЕФНсЙЙМђЪНЮЊ ЃЌDЕФКЫДХЙВеёЧтЦзЭМжаНЋГіЯж жжЗхЁЃ
ЃЈ3ЃЉGжаЫљКЌЙйФмЭХЕФУћГЦЮЊЃК
ЃЈ4ЃЉBЁЊCЕФЗДгІРраЭЪЧ ЃЛCЁњDЕФЗДгІРраЭ ЁЃ
aЃЎЯћШЅЗДгІ bЃЎМгГЩЗДгІcЃЎЛЙдЗДгІ dЃЎѕЅЛЏЗДгІ
ЃЈ5ЃЉаДГіЯТСазЊЛЏЕФЛЏбЇЗНГЬЪН
ЂйAЁњB
ЂкCЁњE
ЂлGгыNaOHШмвКЗДгІ
ЃЈ6ЃЉMЪЧFЕФЭЌЯЕЮяЃЌЦфЯрЖдЗжзгжЪСПБШF tJJЁЂ14ЃЌаДГіТњзуЬѕМўЕФMЕФЫљгаПЩФмЕФНсЙЙЃК
ВщПДД№АИКЭНтЮі>>
АйЖШжТаХ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com