
����Ŀ����һ��������ܱ������У��������»�ѧ��Ӧ��CO2(g)��H2(g)![]() CO(g)��H2O(g)���仯ѧƽ�ⳣ��K���¶�T�Ĺ�ϵ���±���
CO(g)��H2O(g)���仯ѧƽ�ⳣ��K���¶�T�Ĺ�ϵ���±���
T/�� | 700 | 800 | 830 | 1000 | 1200 |
K | 0.6 | 0.9 | 1.0 | 1.7 | 2.6 |
�ش��������⣺
(1)�÷�ӦΪ____________��Ӧ(����ȡ��������ȡ�)��
(2)��ʹ�÷�Ӧ�ķ�Ӧ����������ƽ��������Ӧ�����ƶ�����___________��
a.��ʱ�����CO���� b.�ʵ������¶�
c.����CO2��Ũ�� d.ѡ���Ч����
(3)ij�¶ȣ�ƽ��Ũ�ȷ�����ʽ�� c(CO2)��c(H2)��c(CO)��c(H2O)�����жϴ�ʱ���¶�Ϊ______�档
(4)����(3)�������¶��£���1L���ܱ������У�����2molCO2��3molH2��ַ�Ӧ��ƽ��ʱ��H2�����ʵ���Ϊ__________��CO2�����ʵ���Ϊ__________��
a.����1.0mol b.����1.0mol c.����0.5mol��С��1.0mol d.��ȷ��
(5)�����о��������ڳ��¡���ѹ�����������£�N2�ڴ���(��������Fe2O3��TiO2)������ˮ�������з�Ӧ��2N2(g)��6H2O(l)![]() 4NH3(g)��3O2(g)����H��akJ��mol-1
4NH3(g)��3O2(g)����H��akJ��mol-1
��һ���о�NH3���������¶ȵĹ�ϵ����ѹ�´ﵽƽ��ʱ��ò���ʵ�����������
T/K | 303 | 313 | 323 |
NH3������/(10-6 mol) | 4.8 | 5.9 | 6.0 |
�ٴ˺ϳɷ�Ӧ��
����֪��N2(g)��3H2(g)=2NH3(g)����H=-92.4kJ��mol-1
2H2(g)��O2(g)=2H2O(l)����H=-571.6kJ��mol-1
�����µ�����ˮ��Ӧ���ɰ������������Ȼ�ѧ����ʽΪ��_____________________________��
���𰸡����� bc 830 b c �� �� 2N2(g)��6H2O(l)===4NH3(g)��3O2(g) ��H����1530kJ��mol-1
��������
(1)ͼ�����ݷ�����ƽ�ⳣ�����¶���������˵���¶�����ƽ��������У�����Ӧ�����ȷ�Ӧ��
(2)�÷�ӦCO2(g)��H2(g)![]() CO(g)��H2O(g)�����ȷ�Ӧ���ҷ�Ӧ����������������䡣Ϊ��ʹ�÷�Ӧ�ķ�Ӧ����������ƽ��������Ӧ�����ƶ������������¶Ȼ�����Ӧ��CO2��Ũ�ȣ�
CO(g)��H2O(g)�����ȷ�Ӧ���ҷ�Ӧ����������������䡣Ϊ��ʹ�÷�Ӧ�ķ�Ӧ����������ƽ��������Ӧ�����ƶ������������¶Ȼ�����Ӧ��CO2��Ũ�ȣ�
(3)ij�¶ȣ�ƽ��Ũ�ȷ�����ʽ�� c(CO2)��c(H2)��c(CO)��c(H2O)����K= ![]() =1��
=1��
(4)�С�����ʽ�����
(5) �١�G=��H-T��S�������G<0����Ӧ�����Է����У�
��a N2(g)��3H2(g)=2NH3(g)����H=-92.4kJ��mol-1��b2H2(g)��O2(g)=2H2O(l) ��H=-571.6kJ��mol-1�����ݸ�˹����2a-3b�ɵ�2N2(g)��6H2O(l)===4NH3(g)��3O2(g)��
(1)ͼ�����ݷ�����ƽ�ⳣ�����¶���������˵���¶�����ƽ��������У�����Ӧ�����ȷ�Ӧ��
(2)�÷�ӦCO2(g)��H2(g)![]() CO(g)��H2O(g)�����ȷ�Ӧ���ҷ�Ӧ����������������䡣Ϊ��ʹ�÷�Ӧ�ķ�Ӧ����������ƽ��������Ӧ�����ƶ������������¶Ȼ�����Ӧ��CO2��Ũ�ȣ��������ܸı䷴Ӧ���̣���ʱ�����CO������Ȼ��ʹ��Ӧ�����ƶ���������ʹ�÷�Ӧ�ķ�Ӧ��������ѡbc��
CO(g)��H2O(g)�����ȷ�Ӧ���ҷ�Ӧ����������������䡣Ϊ��ʹ�÷�Ӧ�ķ�Ӧ����������ƽ��������Ӧ�����ƶ������������¶Ȼ�����Ӧ��CO2��Ũ�ȣ��������ܸı䷴Ӧ���̣���ʱ�����CO������Ȼ��ʹ��Ӧ�����ƶ���������ʹ�÷�Ӧ�ķ�Ӧ��������ѡbc��
(3)ij�¶ȣ�ƽ��Ũ�ȷ�����ʽ�� c(CO2)��c(H2)��c(CO)��c(H2O)����K= ![]() =1���ɱ���֪���¶�Ϊ830�棻
=1���ɱ���֪���¶�Ϊ830�棻
(4) CO2(g)��H2(g)![]() CO(g)��H2O(g)
CO(g)��H2O(g)
��ʼ��mol��2 3 0 0
ת����mol��x x x x
ƽ�⣨mol��2-x 3-x x x
K=![]() =
=![]() =1�����x=1.2mol����ƽ��ʱH2�����ʵ���Ϊ3mol-1.2mol=1.8mol������1.0mol����ѡb��CO2�����ʵ���Ϊ2mol-1.2mol=0.8mol������0.5mol��С��1.0mol����ѡc��
=1�����x=1.2mol����ƽ��ʱH2�����ʵ���Ϊ3mol-1.2mol=1.8mol������1.0mol����ѡb��CO2�����ʵ���Ϊ2mol-1.2mol=0.8mol������0.5mol��С��1.0mol����ѡc��
(5) ���ɱ����֪�������¶ȣ�NH3����������Ӧ�����ƶ���˵���÷�Ӧ���ȣ���H��akJ��mol-1��0����a��0���÷�Ӧ���Է���������H��0����G=��H-T��S�������G<0 ��Ӧ�����Է����У��ʡ�S��0��
��a N2(g)��3H2(g)=2NH3(g)����H=-92.4kJ��mol-1��b2H2(g)��O2(g)=2H2O(l) ��H=-571.6kJ��mol-1�����ݸ�˹����2a-3b�ɵ�2N2(g)��6H2O(l)===4NH3(g)��3O2(g) ��H= -92.4kJ��mol-1��2-3����-571.6kJ��mol-1��=��1530 kJ��mol-1���ʴ�Ϊ��2N2(g)��6H2O(l)=4NH3(g)��3O2(g) ��H����1530kJ��mol-1��


 ��Ǭ����������ҵ���ּ����ӱ����������ϵ�д�
��Ǭ����������ҵ���ּ����ӱ����������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ӦC2H6(g)![]() C2H4(g)+H2(g) ��H>0����һ�����������ܱ������дﵽƽ�⡣���и����ʩ�У������������ƽ��ת���ʵ���( )
C2H4(g)+H2(g) ��H>0����һ�����������ܱ������дﵽƽ�⡣���и����ʩ�У������������ƽ��ת���ʵ���( )
A. ���������ݻ�B. ���߷�Ӧ�¶�
C. �������������D. ������ͨ���������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ������Ҫ����0.50 mol/LNaCl��Һ480 mL�������в������������ʵ������֣���ʹ��������������
(1)ѡ����������ɱ�ʵ��������������У�������ƽ(��ȷ��0.1 g)��ҩ�ס��ձ�����������________��________�Լ�����������Ƭ��ֽ��
(2)������������Ƹ���Һ���ȡNaCl����________g��
(3)�ܽ⡢��ȴ���ò�ʵ������Ҫʹ�ò�������Ŀ����___________��
(4)ת�ơ�ϴ�ӡ�
(5)������ƿ�м�������ˮ��ֱ��Һ����̶���Լ1-2����ʱ������___________�μ�����ˮ��Һ����̶������С��Ǻ�ƿ����ҡ�ȡ������ˮʱҺ�泬���̶��ߣ���ʹ��õ���ҺŨ��___________���ƫ�ߡ�����ƫ�͡�����Ӱ�족����
(6)����õ���Һ����һ��ʱ�����ָ�����Լ�ƿ�������ñ�ǩ��ע�����Ƶ�ʱ�䡢��Һ���Ƽ�Ũ�ȡ�
(7)�����ƹ����У�ijѧ���۲춨��ʱҺ�������ͼ��ʾ��������Һ��Ũ�Ȼ�________(�ƫ�ߡ�����ƫ�͡�����Ӱ�족)��
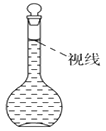
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��Ӧ��2NO2������ɫ��![]() N2O4����ɫ�� ��H��0����100��ʱ����0.40mol NO2�������2L�ܱ������У�ÿ��һ��ʱ��Ը����������ʽ��в������õ����������±���
N2O4����ɫ�� ��H��0����100��ʱ����0.40mol NO2�������2L�ܱ������У�ÿ��һ��ʱ��Ը����������ʽ��в������õ����������±���
ʱ��/s n/mol | 0 | 20 | 40 | 60 | 80 | 100 |
n(NO2) | 0.40 | a | 0.26 | c | d | e |
n(N2O4) | 0.00 | 0.05 | b | 0.08 | 0.08 | 0.08 |
��1��100s�ͷ�Ӧ�������¶ȣ�����������ɫ_________��������dz��������������������������
��2��20��40s�ڣ�v(NO2)=__________mol/(L��s)��100��ʱ�÷�Ӧ��ƽ�ⳣ��K =_____________��
��3����һ������NO2�����ܱ�ע�����У�ͼ���������ѹ��ע�����Ĺ���������������ʱ��ı仯��������ɫԽ�����ԽС��������˵����ȷ����______________
A.b��IJ�����ѹ��ע����
B.c����a����ȣ�c(NO2)����c(N2O4)��С
C.���ܱ�ע����Ϊ������������T(b)��T(c)
D.d��ʱv��������v���棩
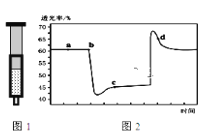
��4����˵����Ӧ2NO2������ɫ��![]() N2O4����ɫ����ƽ�����_________
N2O4����ɫ����ƽ�����_________
A. ��ϵ����ɫ���� B. ���������£�������ܶȲ���
C. 2v����NO2��=v�棨N2O4�� D. ��������ƽ��Ħ����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��N2O��NO��NO2�ȵ��������ǿ�����Ⱦ����е��������β���账��������ŷš�
��1��N2O�Ĵ�����N2O�����������а��������ĸ���������ִ�����ʹN2O�ֽ⡣NH3��O2�ڼ��Ⱥʹ�������������N2O�Ļ�ѧ����ʽΪ________��
��2��NO��NO2�Ĵ������ѳ�ȥN2O������β������NaOH��Һ���գ���Ҫ��ӦΪ
NO+NO2+2OH![]() 2
2![]() +H2O
+H2O
2NO2+2OH![]()
![]() +
+![]() +H2O
+H2O
�����д�ʩ�����β����NO��NO2ȥ���ʵ���________������ĸ����
A���ӿ�ͨ��β��������
B����������Һ�����ķ�ʽ����β��
C������β�������ж��ڲ�������NaOH��Һ
�����պ����Һ��Ũ�����ᾧ�����ˣ��õ�NaNO2���壬�þ����е���Ҫ������________���ѧʽ�������պ��ŷŵ�β���к����ϸߵĵ���������________���ѧʽ����
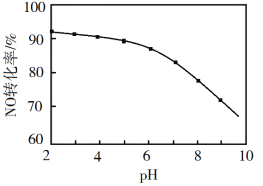
��3��NO���������ա���NaClO��Һ��������β���������β����NO��ȥ���ʡ�����������ͬ��NOת��Ϊ![]() ��ת������NaClO��Һ��ʼpH����ϡ������ڣ��ı仯��ͼ��ʾ��
��ת������NaClO��Һ��ʼpH����ϡ������ڣ��ı仯��ͼ��ʾ��
��������NaClO��Һ�У�HClO����NO����Cl��![]() �������ӷ���ʽΪ________��
�������ӷ���ʽΪ________��
��NaClO��Һ�ij�ʼpHԽС��NOת����Խ�ߡ���ԭ����________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��2013��3�£����ֽ�����ˮ����Ⱦ��Ϊ���أ��������ˮ�����ö��ַ������õķ�ʽ����ˮ�ʴ���������˵���д�����ǣ� ��
A. �ӻ���̿������ˮ��С����������ˮ�ʵķ���������������
B. �ӳ�����ˮ���������������˳�����������
C. ��ϸ����ø�����䷨ȥ��ˮ�еİ����ķ����������
D. �þۺ���������Ϊ��ˮ�����ô��������н������˻�ѧ�仯
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)��֪�������ݣ�
��ѧ�� | H-H | N��N |
����/kJ��mol-1 | 435 | 943 |
��ͼ��N2(g)��H2(g)��Ӧ����1molNH3(g)�����������仯ʾ��ͼ���Ը��ݱ��м�ͼ�����ݼ���N-H�ļ���______________��

(2)�¿���Ϊ�����������ȼ�ϣ���������N2O4��Ӧ����N2��ˮ������
��֪����N2(g)��2O2(g)=N2O4(l) ��H1��-19.5 kJ��mol-1
��N2H4(l)��O2(g)=N2(g)��2H2O(g) ��H2��-534.2 kJ��mol-1
д���º�N2O4��Ӧ���Ȼ�ѧ����ʽ______________________________��
(3)���ñ�״����4.48LO2����N2H4��N2������������ת�Ƶĵ�������Ϊ___________(�����ӵ�������NA��ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ͼ��ʾװ�ÿ��Խ���������CO2ת��Ϊȼ������CO.����˵���У���ȷ����( )
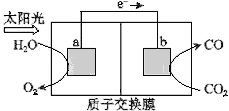
A.��װ�ù���ʱ��H����b������a�����ƶ�
B.��װ����ÿ����1 mol CO��ͬʱ����1 mol O2
C.�缫a���淢����ԭ��Ӧ
D.�ù����ǽ�̫����ת��Ϊ��ѧ�ܵĹ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���л���X�Ǻϳ����ư�֢ҩ����м��壬��ϳɲ���·�����£�

(1)��Ӧ�ٵķ�Ӧ���Լ�����Ϊ________��
(2)��B�Ʊ�X�Ĺ����У��и�����C����(��X��Ϊͬ���칹��)��C�Ľṹ��ʽΪ_____��
(3)�����й�X��˵����ȷ����________��
A�����������ڷ����� B��X�����к���һ������̼ԭ��
C��X�ɷ�����ԭ��Ӧ D�������Ը��������Һ�ɼ�����X��B
(4)д���������һ�ֺ��б�����ͬ���칹��Ľṹ��ʽ_________��
(5)B��һ�������·����Ӿ۷�Ӧ�ɵõ�һ�ָ���ˮ����֬����ṹ��ʽΪ_________��
(6)д��X��NaOH��Һ��Ӧ�Ļ�ѧ����ʽ________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com