
��ͭ���ǹ�ҵ��ͭ����Ҫԭ�ϣ�����Ҫ�ɷ�ΪCuFeS2������һ����Ȼ��ͭ��������ʯ����Ϊ�˲ⶨ�û�ͭ��Ĵ��ȣ�ijͬѧ���������ʵ�飺
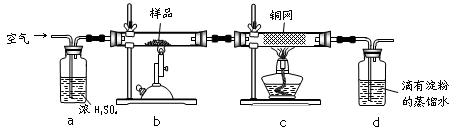
�ֳ�ȡ��ϸ�Ļ�ͭ����Ʒ1.150g���ڿ��������½������գ�����Cu��Fe3O4��SO2���壬ʵ���ȡd����Һ��![]() ������ƿ�У���0.05mol/L������Һ���еζ���������Ϊ0.00mL���ն�����ͼ��ʾ����ش��������⣺
������ƿ�У���0.05mol/L������Һ���еζ���������Ϊ0.00mL���ն�����ͼ��ʾ����ش��������⣺
��1��������Ʒ���õ�����Ϊ_____������Ʒ��ϸ���ٷ�Ӧ����Ŀ����_______��
��2��װ��A��������________��
a�������ڿ�����������ַ�Ӧ b����ȥ�����е�ˮ����
c�������������� d�������ڹ۲��������
��3��������Ӧ����������ͨһ��ʱ��Ŀ�������Ŀ����___________��
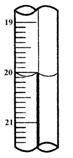
��4���ζ�ʱ��������Һ�������Ϊ_________mL���жϵζ��Ѵ��յ��������_______��
ͨ�������֪���û�ͭ��Ĵ���Ϊ________��
��5��������ͼװ���������ʵ��װ��d��ͬ�����Դﵽʵ��Ŀ�ĵ���____�������ţ�
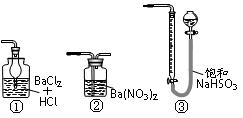
��6������ԭװ��d�е���Һ��ΪBa(OH)2����õĻ�ͭ�����Ϊ��1%������ʵ���������ȷ�����ܵ�ԭ����Ҫ��__________________________________________________��
 ��ʱѵ���������������ϵ�д�
��ʱѵ���������������ϵ�д� �ƸԾ���Ȥζ����ϵ�д�
�ƸԾ���Ȥζ����ϵ�д� ����С����ҵ��ϵ�д�
����С����ҵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ��ʯ���� | ��ͭ�� | ��ͭ�� | ��ͭ�� | ��ȸʯ |
| ��Ҫ�ɷ� | CuFeS2 | Cu5FeS4 | Cu2S | CuCO3?Cu��OH��2 |
| ||
| ѡ�� | ������ | ������ | �ж� |
| A | ͭ�̵����ɷ��Ǽ���ͭ | ����ϡ�����ͭ�������ͭ�� | ��ԣ���ԣ��� |
| B | ͭ�����γ����ܵ�����Ĥ | ͭ��������ʢ��Ũ���� | ��ԣ���ԣ��� |
| C | ����ͭ���� | ����ͭ���ϵ������ڳ�ʪ�����в������� | ��ԣ���ԣ��� |
| D | ��ɫ����ͭ��������ת��Ϊ��ɫ����ͭ��ĩ�������仯 | ����ͭ��Һ��������Ӿ�ص������� | �������ԣ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(08��㶫��)ͭ����Ȼ������ڶ��ֿ�ʯ�У��磺
��ʯ���� | ��ͭ�� | ��ͭ�� | ��ͭ�� | ��ȸʯ |
��Ҫ�ɷ� | CuFeS2 | Cu5FeS4 | Cu2S | CuCO3?Cu(OH)2 |
��ش��������⣺
��1���ϱ�����ͭ�������У�ͭ�������ٷֺ�����ߵ���������������
��2����ҵ���Ի�ͭ��Ϊԭ�ϡ����û�������������ͭ���ù��յ��м���̻ᷢ����Ӧ��2Cu2O+Cu2S![]() 6Cu+SO2������Ӧ����������������������
6Cu+SO2������Ӧ����������������������
��3��SO2β��ֱ���ŷŵ���������ɻ�����Ⱦ�ĺ����������������������β���ɵõ��м�ֵ�Ļ�ѧƷ��д������1�����1���ε�����������������
��4����ͭ��������õ��Ĵ�ͭ������Fe��Ag��Au�Ƚ������ʣ����һ�����õ�ⷨ���ơ��������ͭ���õ���ͭ�Ĵ�������������������
��5���±��У��Գ��������ȷ�Լ������������ϵ���ж϶���ȷ����������������ĸ����
ѡ�� | ������ | ������ | �ж� |
A | ͭ�̵����ɷ��Ǽ���ͭ | ����ϡ�����ͭ�������ͭ�� | ��ԣ��ԣ��� |
B | ͭ�����γ����ܵ�����Ĥ | ͭ��������ʢ��Ũ���� | ��ԣ��ԣ��� |
C | ����ͭ���� | ����ͭ���ϵ������ڳ�ʪ�����в������� | ��ԣ��ԣ��� |
D | ��ɫ����ͭ��������ת��Ϊ��ɫ����ͭ��ĩ�������仯 | ����ͭ��Һ��������Ӿ�ص������� | ������ԣ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
![]()
![]() ͭ����Ȼ������ڶ��ֿ�ʯ�У��磺
ͭ����Ȼ������ڶ��ֿ�ʯ�У��磺
| ��ʯ���� | ��ͭ�� | ��ͭ�� | ��ͭ�� | ��ȸʯ |
| ��Ҫ�ɷ� | CuFeS2 | Cu5FeS4 | Cu2S | CuCO3��Cu(OH)2 |
��ش��������⣺
��1���ϱ�����ͭ�������У�ͭ�������ٷֺ�����ߵ���������������
��2����ҵ���Ի�ͭ��Ϊԭ�ϡ����û�������������ͭ���ù��յ��м���̻ᷢ����Ӧ��2Cu2O+Cu2S![]() 6Cu+SO2������Ӧ����������������������
6Cu+SO2������Ӧ����������������������
��3��SO2β��ֱ���ŷŵ���������ɻ�����Ⱦ�ĺ����������������������β���ɵõ��м�ֵ�Ļ�ѧƷ��д������1�����1���ε�����������������
��4����ͭ��������õ��Ĵ�ͭ������Fe��Ag��Au�Ƚ������ʣ����һ�����õ�ⷨ���ơ���д����ͭ���õ���ͭ�ĵ缫��Ӧʽ������������ ���������� ��
��5���±��У��Գ��������ȷ�Լ������������ϵ���ж϶���ȷ����������������ĸ����
| ѡ�� | ������ | ������ | �ж� |
| A | ͭ�̵����ɷ��Ǽ���ͭ | ����ϡ�����ͭ�������ͭ�� | ��ԣ���ԣ��� |
| B | ͭ�����γ����ܵ�����Ĥ | ͭ��������ʢ��Ũ���� | ��ԣ���ԣ��� |
| C | ����ͭ���� | ����ͭ���ϵ������ڳ�ʪ�����в������� | ��ԣ���ԣ��� |
| D | ��ɫ����ͭ��������ת��Ϊ��ɫ����ͭ��ĩ�������仯 | ����ͭ��Һ��������Ӿ�ص������� | �������ԣ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��11�֣���08��㶫��ѧ��22��
ͭ����Ȼ������ڶ��ֿ�ʯ�У��磺
| ��ʯ���� | ��ͭ�� | ��ͭ�� | ��ͭ�� | ��ȸʯ |
| ��Ҫ�ɷ� | CuFeS2 | Cu5FeS4 | Cu2S | CuCO3��Cu(OH)2 |
��ش��������⣺
��1���ϱ�����ͭ�������У�ͭ�������ٷֺ�����ߵ���������������
��2����ҵ���Ի�ͭ��Ϊԭ�ϡ����û�������������ͭ���ù��յ��м���̻ᷢ����Ӧ��2Cu2O+Cu2S![]() 6Cu+SO2������Ӧ����������������������
6Cu+SO2������Ӧ����������������������
��3��SO2β��ֱ���ŷŵ���������ɻ�����Ⱦ�ĺ����������������������β���ɵõ��м�ֵ�Ļ�ѧƷ��д������1�����1���ε�����������������
��4����ͭ��������õ��Ĵ�ͭ������Fe��Ag��Au�Ƚ������ʣ����һ�����õ�ⷨ���ơ��������ͭ���õ���ͭ��ԭ����������������
��5���±��У��Գ��������ȷ�Լ������������ϵ���ж϶���ȷ����������������ĸ����
| ѡ�� | ������ | ������ | �ж� |
| A | ͭ�̵����ɷ��Ǽ���ͭ | ����ϡ�����ͭ�������ͭ�� | ��ԣ���ԣ��� |
| B | ͭ�����γ����ܵ�����Ĥ | ͭ��������ʢ��Ũ���� | ��ԣ���ԣ��� |
| C | ����ͭ���� | ����ͭ���ϵ������ڳ�ʪ�����в������� | ��ԣ���ԣ��� |
| D | ��ɫ����ͭ��������ת��Ϊ��ɫ����ͭ��ĩ�������仯 | ����ͭ��Һ��������Ӿ�ص������� | �������ԣ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������2010������ڶ����¿���ѧ�Ծ� ���ͣ������
��9�֣�ͭ����Ȼ������ڶ��ֿ�ʯ�У��磺
| ��ʯ���� | ��ͭ�� | ��ͭ�� | ��ͭ�� | ��ȸʯ |
| ��Ҫ�ɷ� | CuFeS2 | Cu5FeS4 | Cu2S | CuCO3��Cu(OH)2 |
 6Cu+SO2������Ӧ����������������������
6Cu+SO2������Ӧ����������������������| ѡ�� | ������ | ������ | �ж� |
| A | ͭ�̵����ɷ��Ǽ���ͭ | ����ϡ�����ͭ�������ͭ�� | ��ԣ���ԣ��� |
| B | ͭ�����γ����ܵ�����Ĥ | ͭ��������ʢ��Ũ���� | ��ԣ���ԣ��� |
| C | ����ͭ���� | ����ͭ���ϵ������ڳ�ʪ�����в������� | ��ԣ���ԣ��� |
| D | ��ɫ����ͭ��������ת��Ϊ��ɫ����ͭ��ĩ�������仯 | ����ͭ��Һ��������Ӿ�ص������� | �������ԣ��� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com