
·ÖĪö £Ø1£©ČōAĪŖ½šŹōĀĮ£¬BĪŖŃõ»ÆĢś£¬ŌņA+B”śC+DĪŖĀĮČČ·“Ó¦£»
£Ø2£©ČōAŹĒŅ»ÖÖÄÜŹ¹ŹŖČóµÄŗģÉ«ŹÆČļŹŌÖ½±äĄ¶µÄĘųĢ壬¼“ĪŖ°±Ęų£¬øĆ·“Ó¦ŹĒ¹¤ŅµÉĻÖĘČ”ĻõĖįµÄÖŲŅŖ·“Ó¦Ö®Ņ»£¬ŌņøĆ·“Ó¦ĪŖ°±µÄ“ß»ÆŃõ»Æ£»
£Ø3£©ČōAŹĒµ»ĘÉ«·ŪÄ©£¬³£ÓĆ×÷¹©Ńõ¼Į£¬CĪŖĒæ¼ī£¬ŌņAĪŖ¹żŃõ»ÆÄĘ£¬øĆ·“Ó¦ĪŖ¹żŃõ»ÆÄĘÓėĖ®µÄ·“Ó¦£»
£Ø4£©ČōA”¢B”¢D¶¼ŹĒÓŠ»ś»ÆŗĻĪļ£¬ĘäÖŠA”¢BŹĒ¼ŅĶ„³ų·æÖŠ³£¼ūµ÷Ī¶Ę·µÄÖ÷ŅŖ³É·Ö£¬ĒŅAµÄĻą¶Ō·Ö×ÓÖŹĮæ±ČB“ó14£¬ŌņAĪŖŅŅĖį£¬BĪŖŅŅ“¼£¬DĪŖŅŅĖįŅŅõ„£¬¾Ż“Ė“šĢā£®
½ā“š ½ā£ŗ£Ø1£©ČōAĪŖ½šŹōĀĮ£¬BĪŖŃõ»ÆĢś£¬ŌņA+B”śC+DĪŖĀĮČČ·“Ó¦£¬øĆ·“Ó¦µÄŅ»ÖÖÓĆĶ¾ŹĒŗø½ÓøÖ¹ģ£¬¹Ź“š°øĪŖ£ŗŗø½ÓøÖ¹ģ£»
£Ø2£©ČōAŹĒŅ»ÖÖÄÜŹ¹ŹŖČóµÄŗģÉ«ŹÆČļŹŌÖ½±äĄ¶µÄĘųĢ壬¼“ĪŖ°±Ęų£¬øĆ·“Ó¦ŹĒ¹¤ŅµÉĻÖĘČ”ĻõĖįµÄÖŲŅŖ·“Ó¦Ö®Ņ»£¬ŌņøĆ·“Ó¦ĪŖ°±µÄ“ß»ÆŃõ»Æ£¬·“Ó¦µÄ»Æѧ·“Ó¦·½³ĢŹ½ĪŖ4NH3+5O2$\frac{\underline{“߻ƼĮ}}{”÷}$4NO+6H2O£¬
¹Ź“š°øĪŖ£ŗ4NH3+5O2$\frac{\underline{“߻ƼĮ}}{”÷}$4NO+6H2O£»
£Ø3£©ČōAŹĒµ»ĘÉ«·ŪÄ©£¬³£ÓĆ×÷¹©Ńõ¼Į£¬CĪŖĒæ¼ī£¬ŌņAĪŖ¹żŃõ»ÆÄĘ£¬øĆ·“Ó¦ĪŖ¹żŃõ»ÆÄĘÓėĖ®µÄ·“Ó¦£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ2Na2O2+2H2O=4NaOH+O2”ü£¬
¹Ź“š°øĪŖ£ŗ2Na2O2+2H2O=4NaOH+O2”ü£»
£Ø4£©ČōA”¢B”¢D¶¼ŹĒÓŠ»ś»ÆŗĻĪļ£¬ĘäÖŠA”¢BŹĒ¼ŅĶ„³ų·æÖŠ³£¼ūµ÷Ī¶Ę·µÄÖ÷ŅŖ³É·Ö£¬ĒŅAµÄĻą¶Ō·Ö×ÓÖŹĮæ±ČB“ó14£¬ŌņAĪŖŅŅĖį£¬BĪŖŅŅ“¼£¬DĪŖŅŅĖįŅŅõ„£¬øĆ·“Ó¦µÄ»Æѧ·“Ó¦·½³ĢŹ½ĪŖCH3COOH+C2H5OH$”ś_{”÷}^{ÅØH_{2}SO_{4}}$ CH3COOC2H5+H2O£¬øĆ·“Ó¦ĪŖČ”“ś£Øõ„»Æ£©·“Ó¦£¬
¹Ź“š°øĪŖ£ŗCH3COOH+C2H5OH$”ś_{”÷}^{ÅØH_{2}SO_{4}}$ CH3COOC2H5+H2O£»Č”“ś£Øõ„»Æ£©·“Ó¦£®
µćĘĄ ±¾Ģāæ¼²éĮĖĪļÖŹ×Ŗ»Æ¹ŲĻµµÄĶʶĻŗĶÓ¦ÓĆ£¬ÄѶČÖŠµČ£¬“šĢāŹ±×¢Ņā³£¼ūŌŖĖŲ»ÆŗĻĪļÖŖŹ¶µÄĮé»īŌĖÓĆŅŌ¼°ÓŠ»ś»ÆŗĻĪļÖŖŹ¶µÄÓ¦ÓĆ£®


 ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø
ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
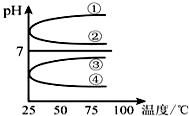 £Ø1£©Ļ”ŹĶ0.1mol•L-1°±Ė®Ź±£¬Ėę×ÅĖ®ĮæµÄŌö¼Ó¶ų¼õŠ”µÄŹĒ¢Ł¢Ś£ØĢīŠ“ŠņŗÅ£©£®
£Ø1£©Ļ”ŹĶ0.1mol•L-1°±Ė®Ź±£¬Ėę×ÅĖ®ĮæµÄŌö¼Ó¶ų¼õŠ”µÄŹĒ¢Ł¢Ś£ØĢīŠ“ŠņŗÅ£©£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ķ¬ĪĀĶ¬Ń¹ĻĀ£¬ĻąĶ¬Ģå»żµÄĪļÖŹ£¬ĖüĆĒµÄĪļÖŹµÄĮæŅ»¶ØĻąµČ | |
| B£® | ČĪŗĪĢõ¼žĻĀ£¬µČĪļÖŹµÄĮæµÄ¶žŃõ»ÆĮņŗĶŅ»Ńõ»ÆĢ¼Ėłŗ¬µÄ·Ö×ÓŹżŅ»¶ØĻąµČ | |
| C£® | 1LŅ»Ńõ»ÆĢ¼ĘųĢåŅ»¶Ø±Č1LŃõĘųµÄÖŹĮ抔 | |
| D£® | µČĢå»ż”¢µČĪļÖŹµÄĮæÅØ¶ČµÄĒæĖįÖŠĖłŗ¬µÄH+ŹżŅ»¶ØĻąµČ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ņ»¶ØĢõ¼žĻĀ£¬6.4 gĶÓė¹żĮæµÄĮņ·“Ó¦£¬×ŖŅʵē×ÓŹżÄæĪŖ0.2NA | |
| B£® | 3molµ„ÖŹFeĶźČ«×Ŗ±äĪŖFe3O4£¬Ź§Č„8NAøöµē×Ó | |
| C£® | ±ź×¼×“æöĻĀ£¬11.2L SO3 ÖŠŗ¬ÓŠ2NAøöŌ×Ó | |
| D£® | ÓĆŹÆ»ŅČéĶźČ«ĪüŹÕ1 mol Cl2Ź±£¬×ŖŅʵē×ӵďżÄæŹĒ2NA |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 ŗĶ
ŗĶ  D”¢35ClŗĶ37Cl
D”¢35ClŗĶ37Cl
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¼ÓČėMgÄܷųöH2µÄČÜŅŗÖŠ£ŗK+”¢Al3+”¢Cl-”¢SO42- | |
| B£® | “ęŌŚ½Ļ¶ąµÄFe3+µÄČÜŅŗÖŠ£ŗHCO3-”¢Cl-”¢SO42- | |
| C£® | Ė®µēĄė²śÉśµÄc£ØOH-£©=1”Į10-10 mol/LµÄČÜŅŗÖŠ£ŗAl3+”¢SO42-”¢NO3-”¢Cl- | |
| D£® | Ź¹¼×»ł³Č±äŗģÉ«µÄČÜŅŗÖŠ£ŗNa+”¢AlO2-”¢NO3-”¢CO32- |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
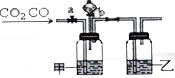 Ä³Ń§ÉśÓĆČēĶ¼×°ÖĆ½ųŠŠCOŗĶCO2»ģŗĻĘųĢåµÄ·ÖĄėŗĶøÉŌļ£®ĘäÖŠaĪŖµÆ»É¼Š£ØæŲÖĘĘųĢåĶعż£©£¬bĪŖ·ÖŅŗĀ©¶·µÄ»īČū£ØøĆ»īČū×÷ÓĆŹĒÓĆÓŚæŲÖĘ·ÖŅŗĀ©¶·ÄŚŅŗĢåµÄĮ÷³öÓė·ń£©£®
Ä³Ń§ÉśÓĆČēĶ¼×°ÖĆ½ųŠŠCOŗĶCO2»ģŗĻĘųĢåµÄ·ÖĄėŗĶøÉŌļ£®ĘäÖŠaĪŖµÆ»É¼Š£ØæŲÖĘĘųĢåĶعż£©£¬bĪŖ·ÖŅŗĀ©¶·µÄ»īČū£ØøĆ»īČū×÷ÓĆŹĒÓĆÓŚæŲÖĘ·ÖŅŗĀ©¶·ÄŚŅŗĢåµÄĮ÷³öÓė·ń£©£®²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com