
(5·Ö)AŹĒµŖ”¢ĒāĮ½ÖÖŌŖĖŲ×é³ÉµÄ»ÆŗĻĪļ”£AÓė°±½į¹¹Ö®¼äµÄ¹ŲĻµĄąĖĘÓŚ¹żŃõ»ÆĒāŗĶĖ®½į¹¹Ö®¼äµÄ¹ŲĻµ”£Ņ»øöA·Ö×Óŗ¬Į½øöµŖŌ×Ó”£ĒėĢīŠ“ŅŌĻĀæÕ°×£ŗ
(1) AµÄ·Ö×ÓŹ½ŹĒ ”£
(2) AµÄĖ®ČÜŅŗ³Ź (ĢīĖį”¢¼ī”¢ÖŠ)ŠŌ£¬1mol A×ī¶ąæÉÓė mol HCl·¢ÉśÖŠŗĶ·“Ó¦ŠĪ³ÉÕżŃĪ”£Š“³öŠĪ³ÉµÄŃĪÖŠŗ¬ÓŠµŖŌ×ӵĥė×ӵĵē×ÓŹ½£ŗ ”£
(3) AŌŚŃõ»Æ»¹Ō·“Ó¦ÖŠŅ²Óė¹żŃõ»ÆĒāĻąĖĘ£¬¼ČæÉ×÷Ńõ»Æ¼Į£¬ÓÖæÉ×÷»¹Ō¼Į”£Ēė“Ó»ÆŗĻ¼ŪµÄ½Ē¶Č·ÖĪö²śÉśÕāÖÖĒéæöµÄŌŅņ£ŗ ”£

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 Ӣ
”¢ £ØČĪŠ“Į½ÖÖ£©
£ØČĪŠ“Į½ÖÖ£© ”¢
”¢ £ØČĪŠ“Į½ÖÖ£©
£ØČĪŠ“Į½ÖÖ£©



| ||
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
 °×ÖŹŗ¬Į棬µ¼ÖĀŠķ¶ąÓ¤Ó׶łÉö½įŹÆ£®
°×ÖŹŗ¬Į棬µ¼ÖĀŠķ¶ąÓ¤Ó׶łÉö½įŹÆ£®








| ·“Ó¦Īļ |
| ·“Ó¦Ģõ¼ž |
| ·“Ó¦Īļ |
| ·“Ó¦Ģõ¼ž |


²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012ÄźĘÕĶØøßµČѧŠ£ÕŠÉśČ«¹śĶ³Ņ»æ¼ŹŌĄķ×Ū»Æѧ²æ·Ö£ØĖēؾķ“ų½āĪö£© ĢāŠĶ£ŗŹµŃéĢā
(17·Ö)¼×”¢ŅŅĮ½øöŃŠ¾æŠŌѧĻ°Š”×éĪŖ²ā¶Ø°±·Ö×ÓÖŠµŖ”¢ĒāŌ×ÓøöŹż±Č£¬Éč¼ĘČēĻĀŹµŃéĮ÷³Ģ£ŗ
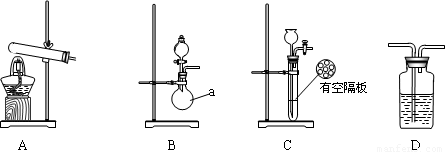
ŹµŃéÖŠ£¬ĻČÓĆÖʵĵݱĘųÅž”Ļ“ĘųĘæĒ°ĖłÓŠ×°ÖĆÖŠµÄæÕĘų£¬ŌŁĮ¬½ÓĻ“ĘųĘæŗĶĘųĢåŹÕ¼Æ×°ÖĆ£¬Į¢¼“¼ÓČČŃõ»ÆĶ”£·“Ó¦Ķź±Ļŗó£¬ŗŚÉ«µÄŃõ»ÆĶ×Ŗ»ÆĪŖŗģÉ«µÄĶ”£
ĻĀĶ¼A”¢B”¢CĪŖ¼×”¢ŅŅĮ½Š”×éÖĘČ”°±ĘųŹ±æÉÄÜÓƵ½µÄ×°ÖĆ£¬DĪŖŹ¢ÓŠÅØĮņĖįµÄĻ“ĘųĘ攣
¼×Š”×é²āµĆ£¬·“Ó¦Ē°Ńõ»ÆĶµÄÖŹĮæm1g”¢Ńõ»ÆĶ·“Ó¦ŗóŹ£Óą¹ĢĢåµÄÖŹĮæm2g”¢Éś³ÉµŖĘųŌŚ±ź×¼×“æöĻĀµÄĢå»żV1L”£
ŅŅŠ”×é²āµĆ£¬Ļ“ĘųĒ°×°ÖĆDµÄÖŹĮæm3g”¢Ļ“Ęųŗó×°ÖĆDµÄÖŹĮæm4g”¢Éś³É°±ĘųŌŚ±ź×¼×“æöĻĀµÄĢå»żV2L”£
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©Š“³öŅĒĘ÷aµÄĆū³Ę ”£
£Ø2£©¼ģ²éA×°ÖĆĘųĆÜŠŌµÄ²Ł×÷ŹĒ ”£
£Ø3£©¼×”¢ŅŅĮ½Š”×éŃ”ŌńĮĖ²»Ķ¬µÄ·½·ØÖĘČ”°±Ęų£¬Ēė½«ŹµŃé×°ÖƵÄ×ÖÄø±ąŗÅŗĶÖʱøŌĄķĢīŠ“ŌŚĻĀ±ķµÄæÕøńÖŠ”£
| | ŹµŃé×°ÖĆ | ŹµŃéŅ©Ę· | ÖʱøŌĄķ |
| ¼×Š”×é | A | ĒāŃõ»ÆøĘ”¢ĮņĖį”¢ĮņĖįļ§ | ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ¢Ł ”£ |
| ŅŅŠ”×é | ¢Ś | ÅØ°±Ė®”¢ĒāŃõ»ÆÄĘ | ÓĆ»ÆŃ§Ę½ŗāŌĄķ·ÖĪöĒāŃõ»ÆÄʵÄ×÷ÓĆ£ŗ ¢Ū ”£ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012ÄźĘÕĶØøßµČѧŠ£ÕŠÉśČ«¹śĶ³Ņ»æ¼ŹŌĄķ×Ū»Æѧ²æ·Ö£ØĖēؾķ½āĪö°ę£© ĢāŠĶ£ŗŹµŃéĢā
(17·Ö)¼×”¢ŅŅĮ½øöŃŠ¾æŠŌѧĻ°Š”×éĪŖ²ā¶Ø°±·Ö×ÓÖŠµŖ”¢ĒāŌ×ÓøöŹż±Č£¬Éč¼ĘČēĻĀŹµŃéĮ÷³Ģ£ŗ

ŹµŃéÖŠ£¬ĻČÓĆÖʵĵݱĘųÅž”Ļ“ĘųĘæĒ°ĖłÓŠ×°ÖĆÖŠµÄæÕĘų£¬ŌŁĮ¬½ÓĻ“ĘųĘæŗĶĘųĢåŹÕ¼Æ×°ÖĆ£¬Į¢¼“¼ÓČČŃõ»ÆĶ”£·“Ó¦Ķź±Ļŗó£¬ŗŚÉ«µÄŃõ»ÆĶ×Ŗ»ÆĪŖŗģÉ«µÄĶ”£
ĻĀĶ¼A”¢B”¢CĪŖ¼×”¢ŅŅĮ½Š”×éÖĘČ”°±ĘųŹ±æÉÄÜÓƵ½µÄ×°ÖĆ£¬DĪŖŹ¢ÓŠÅØĮņĖįµÄĻ“ĘųĘ攣
¼×Š”×é²āµĆ£¬·“Ó¦Ē°Ńõ»ÆĶµÄÖŹĮæm1g”¢Ńõ»ÆĶ·“Ó¦ŗóŹ£Óą¹ĢĢåµÄÖŹĮæm2g”¢Éś³ÉµŖĘųŌŚ±ź×¼×“æöĻĀµÄĢå»żV1L”£
ŅŅŠ”×é²āµĆ£¬Ļ“ĘųĒ°×°ÖĆDµÄÖŹĮæm3g”¢Ļ“Ęųŗó×°ÖĆDµÄÖŹĮæm4g”¢Éś³É°±ĘųŌŚ±ź×¼×“æöĻĀµÄĢå»żV2L”£
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©Š“³öŅĒĘ÷aµÄĆū³Ę ”£
£Ø2£©¼ģ²éA×°ÖĆĘųĆÜŠŌµÄ²Ł×÷ŹĒ ”£
£Ø3£©¼×”¢ŅŅĮ½Š”×éŃ”ŌńĮĖ²»Ķ¬µÄ·½·ØÖĘČ”°±Ęų£¬Ēė½«ŹµŃé×°ÖƵÄ×ÖÄø±ąŗÅŗĶÖʱøŌĄķĢīŠ“ŌŚĻĀ±ķµÄæÕøńÖŠ”£
|
|
ŹµŃé×°ÖĆ |
ŹµŃéŅ©Ę· |
ÖʱøŌĄķ |
|
¼×Š”×é |
A |
ĒāŃõ»ÆøĘ”¢ĮņĖį”¢ĮņĖįļ§ |
·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ¢Ł ”£ |
|
ŅŅŠ”×é |
¢Ś |
ÅØ°±Ė®”¢ĒāŃõ»ÆÄĘ |
ÓĆ»ÆŃ§Ę½ŗāŌĄķ·ÖĪöĒāŃõ»ÆÄʵÄ×÷ÓĆ£ŗ ¢Ū ”£ |
£Ø4£©¼×Š”×éÓĆĖł²āµĆŹż¾Ż¼ĘĖć³ö°±·Ö×ÓÖŠµŖ”¢ĒāµÄŌ×ÓøöŹżÖ®±ČĪŖ ”£
£Ø5£©ŅŅŠ”×éÓĆĖł²āµĆŹż¾Ż¼ĘĖć³ö°±·Ö×ÓÖŠµŖ”¢ĒāµÄŌ×ÓøöŹż±ČĆ÷ĻŌŠ”ÓŚĄķĀŪÖµ£¬ĘäŌŅņŹĒ
”£ĪŖ“Ė£¬ŅŅŠ”×éŌŚŌÓŠŹµŃéµÄ»ł“”ÉĻŌö¼ÓĮĖŅ»øöװӊijŅ©Ę·µÄŹµŃéŅĒĘ÷£¬ÖŲŠĀŹµŃ锣øł¾ŻŹµŃéĒ°ŗóøĆŅ©Ę·µÄÖŹĮæ±ä»Æ¼°Éś³É°±ĘųµÄĢå»ż£¬µĆ³öĮĖŗĻĄķµÄŹµŃé½į¹ū”£øĆŅ©Ę·µÄĆū³ĘŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com